Table of Contents
ToggleIonization energy is defined as the amount of energy required to remove the most loosely bound electron from an isolated gaseous atom in its ground state to produce a cation. Ionization energy is the smallest potential difference necessary to remove the most loosely bound electron from an isolated gaseous atom. It is also known as ionization potential. This process is represented as:
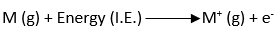
First (1st) ionization energy
The first ionization energy is the energy required to remove the first electron and convert M to M+.
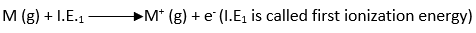
Second (2nd) Ionization energy
The second ionization energy is the energy required to remove the second electron and convert M+ to M2+.
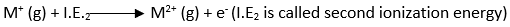
Third (3rd) Ionization energy
The third ionization energy is the energy required to remove the third electron and convert M2+ to M3+.
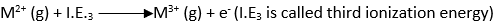
I.E1 < I.E2 < I.E3 < I.E4
Periodic table trends : Ionization energy
Along the period
In general, when we move from left to right in a period, the ionization energy increases with increasing atomic numbers. This tendency is explained by the combined impact of greater nuclear charge and decreasing atomic size, which causes electrons to become increasingly strongly linked to the nucleus. This results in a gradual increase in ionization energies.
Along the group
The ionization energy decreases in a group from top to bottom in a group. A gradual increase in atomic size is due to the addition of a new shell in each element as a result of an increase in nuclear charge. This factor tries to increase the ionization energy along with the group.
Factor affecting Ionization Energy
Atomic Size
Ionization energy decreases with an increase in atomic size. In a bigger atom, outer electrons are held less firmly and it becomes easier to remove the electron.

Magnitude of Nuclear Charge
The ionization energy increases with an increase in nuclear charge. The attractive force is directly proportional to the product of charges of the nucleus and that on electrons, therefore it becomes comparatively difficult to remove the electron.
Screening Effect or Shielding effect
The ionization energy decreases when the shielding or screening effect of the inner electrons increases.
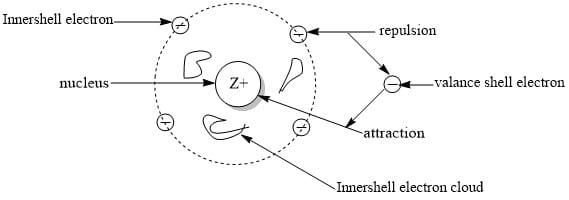
In multi-electron atoms, the electrons in the valance shell are pulled by the nucleus and repelled by electrons of inner shells. Thus, outermost electrons experience less attraction from the nucleus under the combined effect of attractive and repulsive force acting on valance electrons. This effect is called the screening effect.
The actual charge felt by the valance shell electron as a result of this is called an effective nuclear charge (Zeff).
Penetration Effect of Electron based on Shape of Orbital
If the electrons have a greater penetrating ability, they will be closer to the nucleus and will be held firmly by the nucleus. More energy is required to pull out the electron from the s-orbital than p, d, f orbitals. Hence ionization energy decreases in order s> p> d> f orbital.
Electronic Configuration
Half-filled or completely filled orbitals are more stable than others and hence more energy is needed to remove an electron from such atoms. Thus the more stable the electronic configuration, the greater will be the ionization energy.
p6 > d10 > p3 > d 5
Ionizaton energy of K, Li, Mg, Na, Ca, F, O and other Elements
| Elements | Ionization energy(KJ/mol-1) |
| K | 418.9 |
| Li | 520.3 |
| Mg | 738 |
| Be | 899.5 |
| Na | 495.8 |
| Ca | 590 |
| F | 1681.0 |
| O | 1314.0 |
| Ne | 2080.4 |
FAQs / MCQs
What is ionization energy?
Ionization energy is defined as the amount of energy required to remove the most loosely bound electron from an isolated gaseous atom in its ground state to produce a cation.
Which element has the highest ionization energy in the following
Li,Na,K,Rb,Cs
Li has the highest ionization energy (9520.3 KJ/mol-1).
Why does ionization energy increase across a period?
when we move from left to right in a period, the ionization energy increases with increasing atomic numbers.
Why does ionization energy decrease down a group?
A gradual increase in atomic size due to the addition of a new shell in each element as a result increase in nuclear charge. This factor tries to increase the ionization energy along with the group.
Why do noble gases have high ionization energies?
Nobel gases (zero group) show the highest ionization energy in their respective period due to stable electronic configuration (ns2np6)