Table of Contents
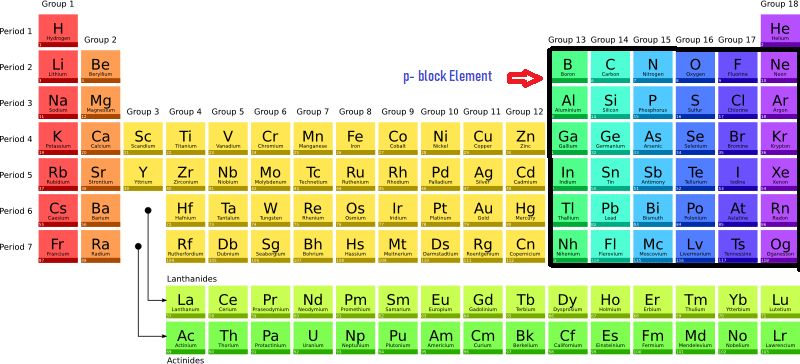
Togglep block elements are those elements in which the last (valence) electron enters the p-subshell of their outermost energy level. This block is situated at the extreme right of the periodic table. The maximum number of electrons that can occur in a set of p orbitals is six since there are three p orbitals. As a result, the periodic table has six groups of p-block elements group (IIIA), 14 (IVA), 15 (VA), 16 (VIA), 17 (VIIA), and 18 (zero) of the periodic table. The electronic configuration of p block elements is ns2np1-6 (except Helium).
Characteristics of p block elements
- p-block elements consist of metals, non-metals, and metalloids.
- Mostly, they form covalent compounds.
- The p-block parts are often shiny and function well as both electrical and thermal conductors.
- P-block elements have considerably greater ionization enthalpies than s-block elements.
- p-block elements do not give characteristic color to the flame.
- They mostly form acidic oxides.
- In a group, the electronegativity decreases from top to bottom and increases in a period from left to right.
- Reducing property decreases from left to right in a period while increasing in a group from top to bottom.
- The non-metallic character steadily increases in the period from left to right. But from top to bottom, non-metallic property reduces in the groups.
- The total amount of valence electrons, or the sum of the ns and np electrons, determines the maximum oxidation state that a p-block element can exhibit. As one proceeds from left to right in the p-block, the number of potential oxidation states increases.
- The allotropy phenomenon is demonstrated by a number of elements of the p-block series. These elements include arsenic, carbon, silicon, phosphorus, sulfur, boron, germanium, tin, and phosphorus.
- Numerous elements in the p-block series, including carbon, silicon, germanium, nitrogen, oxygen, and sulphur, exhibit the catenation property.
p block elements electronic configuration
| Group | 13 (IIIA) | 14 (IVA) | 15 (VA) | 16 (IVA) | 17 (VIIA) | 18 (VIIIA) |
| Electronic Configuration | ns2np1 | ns2np2 | ns2np3 | ns2np4 | ns2np5 | ns2np6 |
| Elements | Boron (B), Aluminum (Al), Gallium (Ga), Indium (In), Thallium (Tl) | Carbon (C), Silicon (Si), Germanium (Ge), Tin (Sn), Lead (Pb) | Nitrogen (N), Phosphorus(P), Arsenic (As), Antimony (Sb), Bismuth (Bi) | Oxygen (O), Sulphur (S), Selenium (Se), Tellurium (Te), Polonium (Po) | Fluorine (F), Chloride (Cl)’ Bromine (Br), Iodine (I), Astatine (At) | Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), Radon (Rn) |
Properties of p block elements
The differences in an element’s inner core have a significant impact on both its chemical and physical properties, including atomic and ionic radii, ionization enthalpy, and several others. The characteristics of the elements in a group of p-block are thus shown to vary significantly.
The initial p-block element is different from the other elements in two key ways:
- The size and any other characteristics that depend on size come first.
- The second distinction only affects the p-block element, which is created as a result of the interactions between d-orbitals and the valence shells of heavier elements.
Uses of p block elements
- Germanium, silicon, arsenic, and gallium are all employed as semiconductors.
- Alum is extensively used for water purification.
- The glass and pottery industries use the boron compound borax.
- Steel’s hardness is increased with boron.
- Numerous applications include the usage of carbon and its compounds.
FAQs
What are p block elements?
p block elements are those elements in which the last electrons enter into the p-subshell of their outermost energy level
How many elements in p block?
There are 35 elements in the p-block.
What is the general electronic configuration of p block elements?
The general electronic configuration of p block elements is ns2np1-6 (except Helium).
Why s and p block elements are called representative elements?
Representative elements are those that have all of their shells completed, with the exception of the outermost shell, which is incomplete. Since the outermost shells are incomplete in all the groups of p block and s block, these elements are called representative elements. Moreover, these are the active, abundant elements that can be found in nature. This is why these elements are known as representative elements.