Table of Contents
ToggleDefinition of phosphazene
Phosphazenes, an important class of phosphonitrilic compounds, are cyclic or linear chain inorganic compounds created by the bonding and repetition of phosphorus and nitrogen atoms with (P=N)n bonds. Phosphazenes are a group of chemicals with organophosphorus phosphorus(V) and a double bond between P and N with two substituents on each P atom. They are known in various structural forms such as cyclic trimers, tetramers, pentamers, hexamers, or higher polymers. The formula of phosphazene is RN=P(NR2)3.
Phosphonitrilic halides are the best-known polymers of the P-N type and are also known as inorganic rubber.
Oxidation state of phosphorus in phosphazene
When compared to trivalent organophosphorus compounds, the phosphorus atom in phosphazene compounds is in its maximum (+5) oxidation state, making them significantly less sensitive to oxygen.
Properties of Phosphazene
- Glass transition temperatures as low as -60 °C are achievable, and qualities like hydrophobicity and oil resistance are what make them useful for land vehicles and aeronautical components. Additionally, biostable biomedical devices have made use of them.
- Phosphazene polymers include brittle glasses and rubbery elastomers. The glass transition temperature is increased by the presence of aryloxy-side groups or by amino-side group hydrogen bonds.
- The rubber-like materials lose their useful properties when in contact with water due to hydrolysis.
- In these ring systems, the P-N bond distances are typically equal or quite close to being equal. They are shorter than the single-bond distances that were expected. These observations imply the existence of numerous bonds and resonance structures between the atoms of P and N.
- The higher polymers are linear in structure.
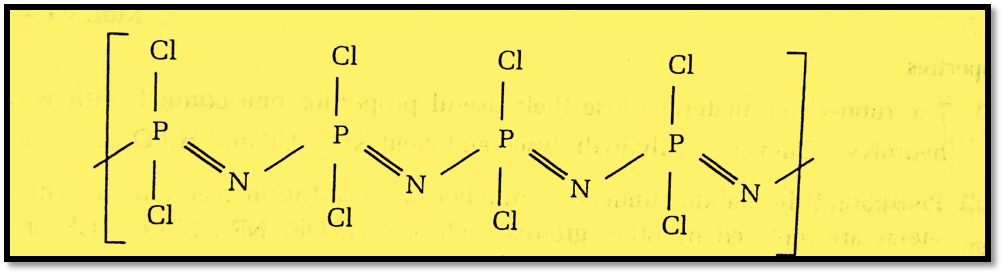
Structure of Phosphazenes
Phosphazenes are a significant class of inorganic compounds that are formed by the bonding and repetition of phosphorus and nitrogen atoms with (P=N)n bonds. They can have cyclic or linear chains. Phosphazene compounds come in a variety of forms, from oligomers to polymers.
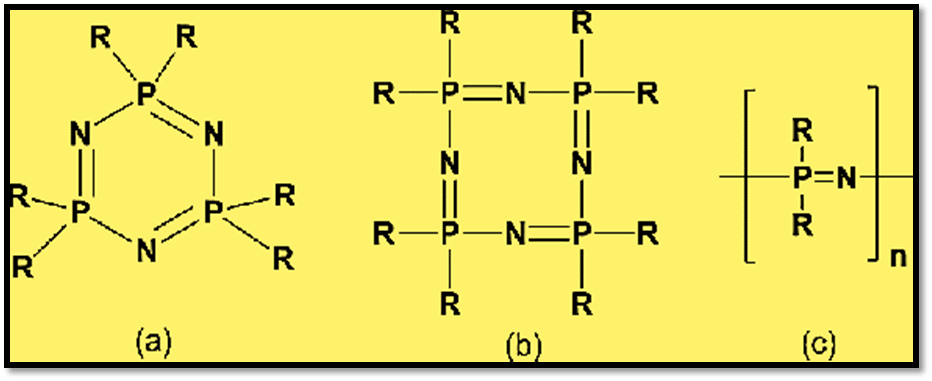
Phosphazenes contain the basic structure unit –X2P= N–.These phosphazenes are also known as iminophosphoranes and phosphine imides. It has an organic side group (R) covalently bound to the phosphorus atoms and an inorganic backbone made up of alternating phosphorus and nitrogen atoms, separated by alternating single and double bonds.
Classification of Phosphazenes
- Polyphosphonitrillic chloride.
- Polydimethoxy phosphazines.
- Polydiethoxy phosphazines.
The history of polyphosphazene is extensive, with cross-linked elastomeric materials (also known as “inorganic rubber”) made of phosphorus and nitrogen being described as early as the 1890s.
Phosphazene preparation
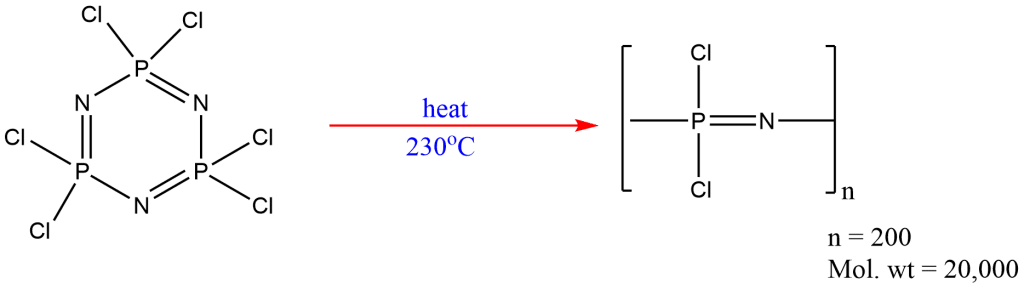
1 . There are several phosphonitrilic chlorides with the general formula (PNCl2)n that are produced when PCl5 and NH4Cl are refluxed in an organic solvent such as tetrachloroethane or chlorobenzene, where n ranges from 3 to 8.

The best known Phosphazenes is the trimer of (PNCl2)3 with a melting point of 114oC and boiling point of 256.5oC.
2. When (PNCl2)3 is heated to about 230oC,, rubber-like chain polymer is formed.
Uses of Phosphazenes
- There are many potential uses for the higher molecular weight Phosphazenes, such as rigid plastics, explained foam, and fibers, since they are waterproof and fireproof and are unaffected by petrol, oil, and solvents.
- They also form flexible plastics, which are useful for fuel hoses and gaskets since they retain their elasticity at low temperatures.
- They are used as slow drug-releasing agents.
- They have high thermal stability. So can be used in place of carbon polymer.
- Additionally, phosphazene is utilized in composite materials and as surface coatings.
Biomedical uses of Phosphazenes
A flexible tool to handle such a trade-off is provided by polyphosphazene, which also offers the best biological interface between the surface of medical implants and surrounding tissues. Modulating the degradation of polyphosphazene can control and regulate the process of tissue regeneration as well as the release of molecules that have been immobilized. Degradable polyphosphazenes have demonstrated promise in their applications as carriers for the controlled release of bioactive and therapeutic molecules.
FAQs/ MCQs
Are Phosphazenes aromatic?
No, Phosphazenes are not aromatic.
What is the basic structural unit in phosphazene?
Phosphazenes contain the basic structure unit –X2P=N–.
Which compound is present in phosphazene?
Generally, Phosphazenes contain organophosphorus ( Phosphorus and nitrogen).
What are Phosphazenes bases?
A nitrogen basic core is doubly bound to pentavalent phosphorus to form phosphazene bases, which are incredibly powerful and uncharged bases.
What are the characteristics of Phosphazenes?
Phosphazenes have the following characteristics: chemical inertness, mechanical strength, high dipole moment, flexibility, biocompatibility, and a wide range of glass transition temperatures.
What is phosphazene?
Organophosphorus compounds known as phosphozenes include phosphorus(V) with a double bond between P and N. RN=P(NR2)3 is the formula for one class of phosphazenes.






