Table of Contents
ToggleDetection of foreign elements is important for locating the class of organic compounds. Organic compounds have been classified into various types, depending upon the elements present in them. All organic compounds are expected to contain carbon and hydrogen, so it is not necessary to test for the presence of these elements.
Detection of Nitrogen, Sulphur, and Halogen
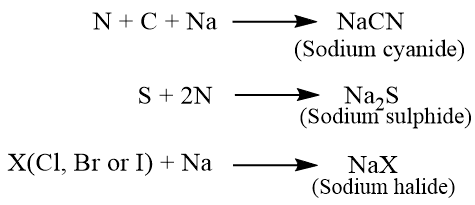
The most important method for the detection of these elements was first introduced by J.L. Lassaign in 1843 and is known as the “Lasseigne’s test.” The theory behind this test is that when organic compounds are fused with sodium, the elements in the compounds are transformed into ionic salts. Thus, nitrogen is transformed into cyanide ions in the presence of carbon, sulphur to sulphide ions, and halogen to halide ions. These ions are then tested in the usual manner.
Sometimes, when nitrogen and sulphur are both present in the compound, and the fusion is incomplete, sodium sulphocyanide is formed.
Preparation of Lassaigne’s filtrate (Sodium extract)
A small piece of sodium (about the size of a pea) is freshly cut and dried between the filter paper folds. It is placed into an ignition tube held by a tong, and the ignition tube is gently and slowly heated by holding it just over the burner’s flame. It will be noticed that the sodium melts to form a shining globule like mercury. At this stage, the ignition tube is removed from the flame and a very small amount (about 0.1 gm for solids and 2-3 drops for liquids) of the organic compound is added to it when an immediate reaction with sodium is observed. The brisk reaction is allowed to subside, and then a small piece of sodium (about the half size of the piece used earlier) is inserted into the ignition tube. The ignition tube is then gently heated by keeping it just above the flame for a few seconds, and as the reaction gains momentum, it is removed from the flame. The process is repeated several times. Finally, it is heated first over a low flame and then strongly for about 5 minutes. The tube will become red hot and probably start melting, but continue heating for the time specified. The red hot ignition tube is plunged into 10 ml distilled water taken in a porcelain dish and crushed with the help of a glass rod. The contents of the porcelain dish are then boiled for a few minutes and filtered. The filtrate is known as Lassaigne’s filtrate or sodium extract.
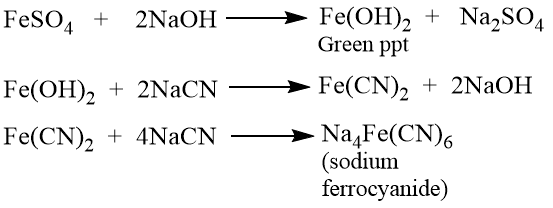
Test for nitrogen
If the sodium extract is not alkaline, it is added to a test tube and made alkaline by adding dilute sodium hydroxide (NaOH). Then it’s mixed with freshly prepared ferrous sulphate (FeSO4) solution, heated, cooled, and a few drops of ferric chloride added. Finally, a few of drops of dil. HCl (hydrochloric acid) is also used. The presence of nitrogen in an organic compound is indicated by the formation of Prussian green or blue color.
The role of HCl is to dissolve the dirty green precipitate of ferrous hydroxide (Fe(OH)2), which otherwise marks the color.
Test for Sulphur
To 2 ml sodium extract add 2-3 drops of freshly prepared sodium sodium nitroprusside solution when a violet or purple colour confirms the presence of sulphur.
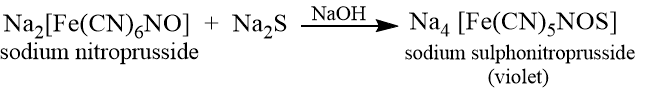
Acidify 2 ml sodium extract with dilute acetic acid and add 1 ml of lead acetate solution when a black precipitate of lead sulphide is produced conforming the presence of sulphur.
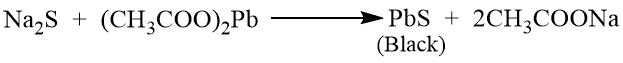
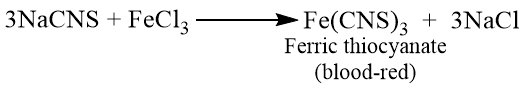
Test for nitrogen and sulphur together
If the fusion has been incompletely carried out and both nitrogen and sulphur are present in the compound, none of the above tests for these elements are shown by the sodium extract. Instead the following test should be performed.
Acidify 2 ml of sodium extract with dilute HCl and add 2-3 drops of FeCl3 solution when red or blood-red colour confirms the presence of both nitrogen and sulphur in the compound.
Test for halogen
If halogens are present in the organic compound then sodium halide is formed.
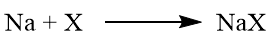
(X = Fluorine, Chlorine, Bromine and Iodine)
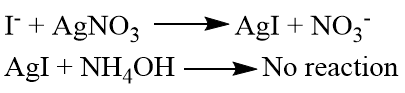
The halide is detected by Silver nitrate (Ag(NO)3) test
For this a little sodium extract is taken in a test tube and few drops of nitric acid is added and boiled and cooled and finally few drops of aqueous silver nitrate is added:
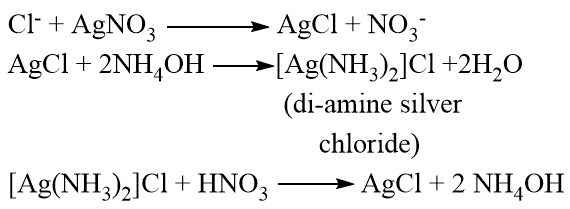
If chlorine is present in the organic compound, then white precipitate(ppt) is obtained which is soluble in NH4OH(ammonium hydroxide) and reappears on adding HNO3 (Nitric acid)
If bromine is present in the organic compound then pale yellow precipitate(ppt) is obtained which is sparingly soluble in ammonium hydroxide (NH4OH).
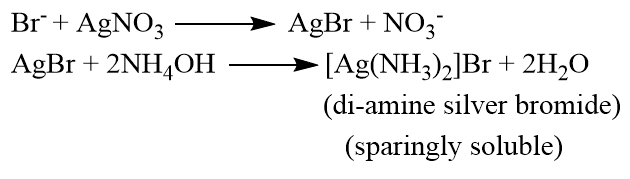
If iodine is present in the organic compound then yellow ppt is obtained which is insoluble in (NH4OH).