Table of Contents
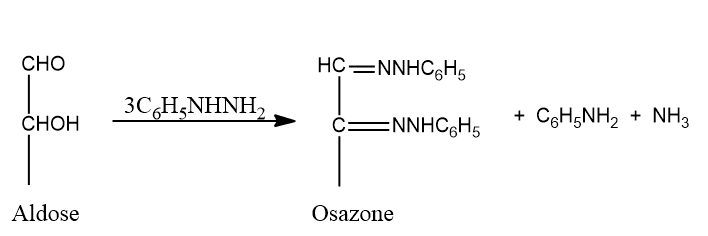
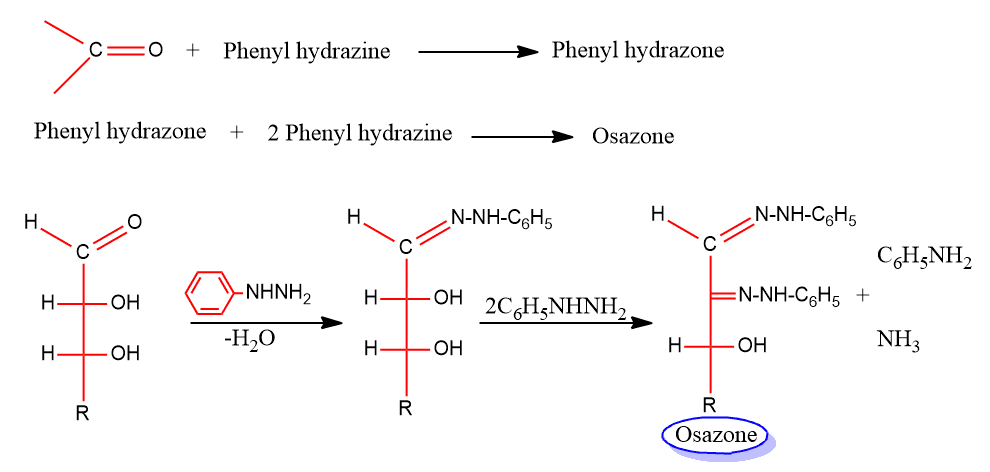
ToggleOsazone is the derivative of carbohydrates that are formed by the reaction of carbohydrates with phenylhydrazine. As aldehyde, aldoses react with phenylhydrazine to form phenylhydrazones. In 1875, Emil Fischer found that reducing benzene diazonium chloride by sulfur dioxide yields phenylhydrazine.
Fischer reported that the phenylhydrazine he had discovered could be used as a powerful tool in the study of carbohydrates. If an excess of phenylhydrazine is used, the reaction proceeds further to yield products known as osazones, Which contain two phenylhydrazine residues per molecule a third molecule of the reagent is turned into aniline and ammonia.
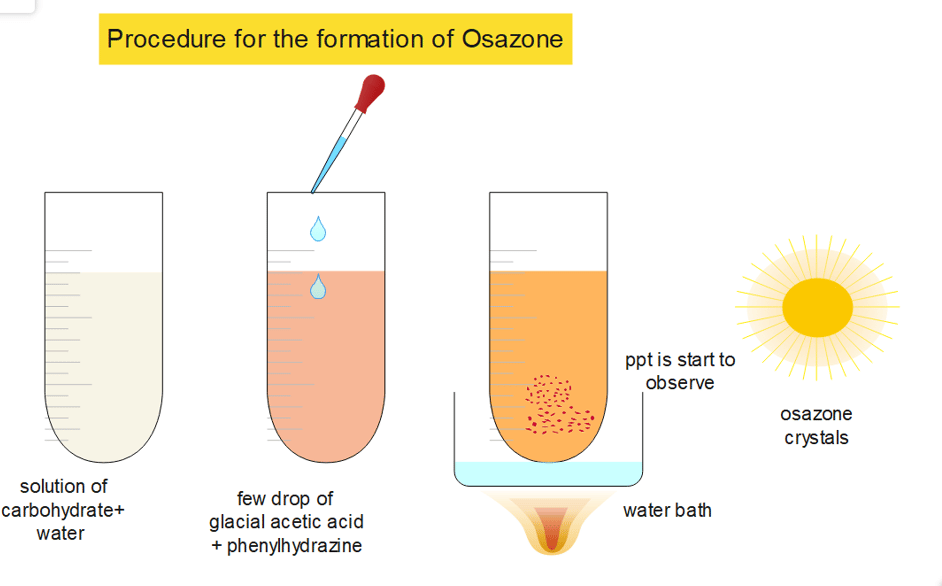
Osazone formation: easy procedure
- An aqueous solution of around 5 mL of carbohydrate sample is taken in a clean and dry boiling tube.
- To the above solution, 10 drops of glacial acetic acid and approx 5 drops of phenylhydrazine are added.
- The solution is shaken well and the mixture is then kept in boiling water (water bath).
- Now, time is noted at the various time interval for the formation of osazone. ( every carbohydrate has a different time for the formation of its osazone)
- After the formation of osazone, the ppt is washed with water, it should be recrystallized from hot water for better purity and for further analyzing purposes.
- Then, it can be observed under a microscope for clear morphological structure.
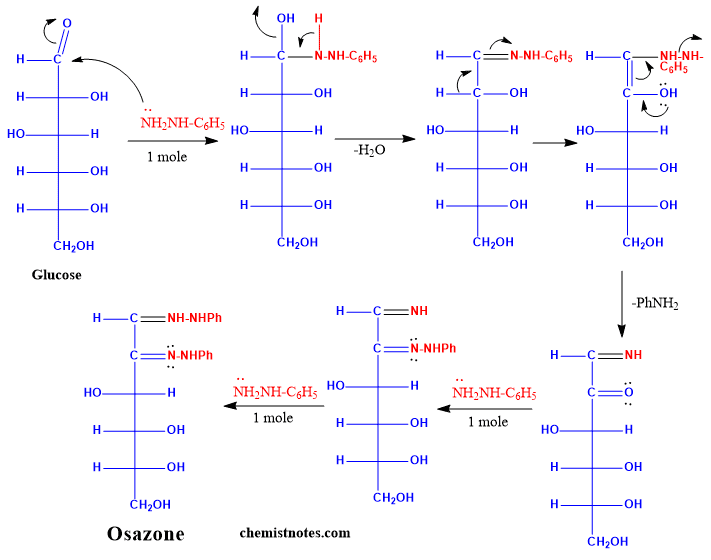
Osazone formation mechanism
One of the difficulties of working with carbohydrates is their tendency to form syrups. Treatment with phenylhydrazine converts carbohydrates into solid osazones, which are readily isolated and purified, and can be identified by characteristic crystalline forms.
Fischer found osazone formation to be useful not only in identifying carbohydrates but also this was much more important in determining their configurations. For example; the two diastereoisomeric aldohexoses (+)-glucose and (+) mannose yields the same osazone. Osazone formation destroys the configuration of C-2 of aldose but does not affect the configuration of the rest of the molecule. It, therefore, follows that (+)-glucose and (+)-mannose differs only in the configuration of C-3, C-4, and C-5. We can see that whatever the configuration of either of these compounds is established, the configuration of the other is immediately known through this osazone relationship.
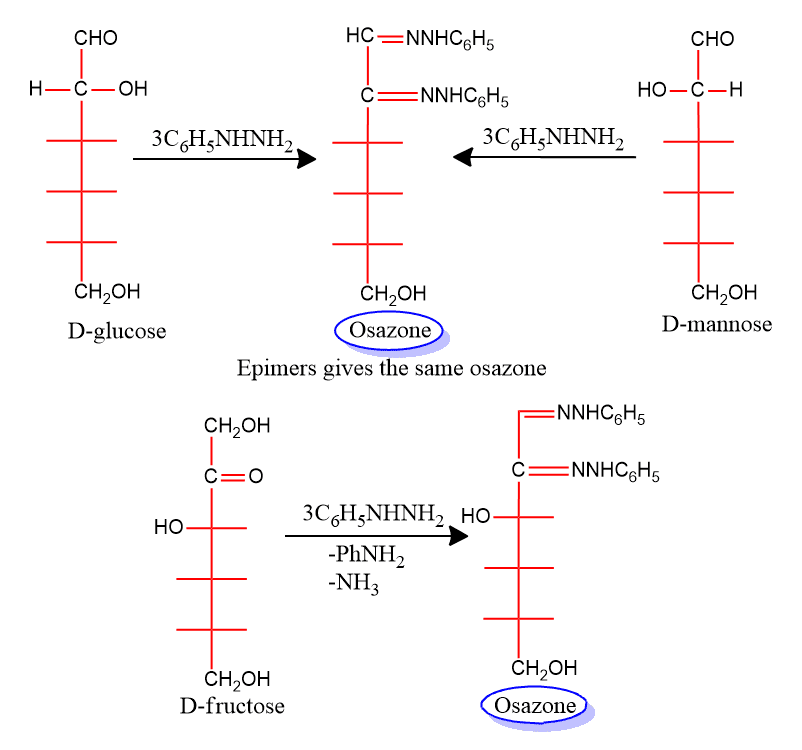
A pair of diastereomeric aldoses that differ only in the configuration of C-2 are called epimers.
Mechanism of osazone formation
The proposed mechanism of osazone formation is given below: