Table of Contents
ToggleThermogravimetric analysis, also known as TGA is a technique frequently used in thermal analysis in which a change in the weight of a substance is recorded as a function of temperature or time. Composition, purity, decomposition processes, decomposition temperatures, and absorbed moisture content are among the characteristics and behavior that can be measured by TGA.
TGA is one of the best methods for determining the thermal characteristics of substances including chemicals, pharmaceuticals, and materials made of plastics, elastomers, and thermosets as well as mineral compounds and ceramics.
Types of thermogravimetric analysis TGA
Thermogravimetric methods are categorized into three types:
- Static (isothermal) thermogravimetry: Such a method involves analysis in which the sample weight is recorded as a function of time at a constant temperature.
- Quasistatic thermogravimetry: In this type of analysis, the sample is heated to constant weight at increasing temperature.
- Dynamic thermogravimetry: The sample is heated in a condition where the temperature is rising or falling at a predefined rate, usually linearly. Most studies using the thermal technique of analysis are typically conducted using dynamic thermogravimetric analysis.
Thermogravimetric analysis Principle
TGA gives a quantitative evaluation of any weight changes brought on by thermally induced transition. The method involves heating a sample at a controlled rate i.e. for a substance with a known initial weight, the temperature is raised steadily, and at various time intervals, the weight changes are recorded as a function of temperature. The results may be presented in the following two ways:
- as a TG curve (thermogravimetric curve) in which the weight change is recorded as a function of temperature or time
- as a TG curve derivative (DTG), where the TG curve’s first derivative is plotted against either temperature or time. The graph plotted is known as the pyrolysis curve.
Due to the distinct order of physical transitions and chemical reactions that take place over specified temperature ranges, the TG curve is unique to a given substance or material. Because of physical changes and the building and breaking of chemical bonds at high temperatures, weight changes result. In either inert or reactive atmospheres, TG typically operates in the 1200oC temperature range. The molecular structure frequently affects the thermally induced reaction rates.
A typical TG curve for CuSO4.5H2O can be shown as:
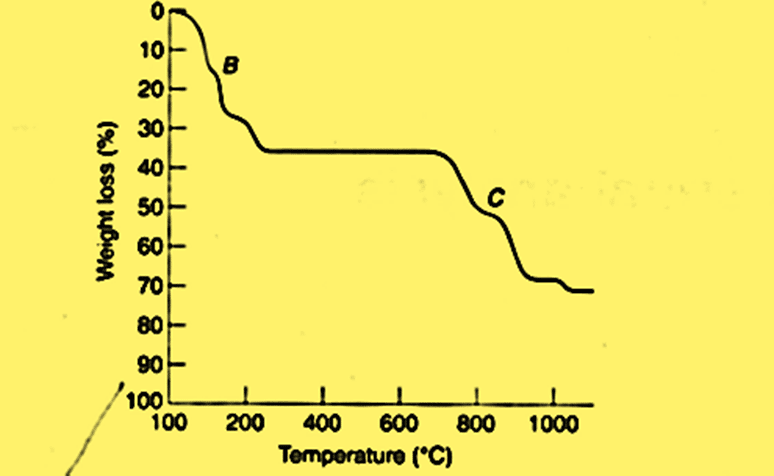
The graph presented here can be categorized into horizontal and vertical portions. The horizontal portions signify regions where there is no weight loss, while the curved region in the vertical portion indicates weight loss. The calculations on compound stoichiometry can be done at any temperature since the TG curve is a quantitative equation.
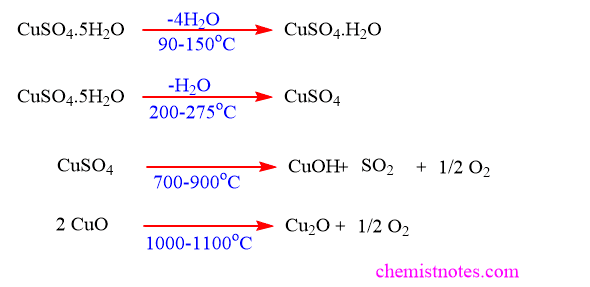
CuSO4.5H2O consists of four distinct regions of decomposition as illustrated below:
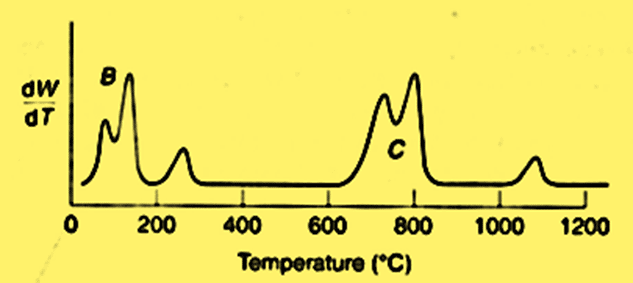
A derivative thermogravimetric (DTG) curve is produced by plotting the weight change over time (dW/dT) vs temperature. When there is no weight loss, dW/DT is equal to zero in the DTG curve. A maximum slope on the TG curve is represented by the derivative curve’s peak. A shift in slope occurs on the TG curve when dW/dT is minimum but not zero, which is known as an inflection.
Thermogravimetric analysis instrumentation
The fundamental instrument needed for TG is a precision balance with a furnace designed to linearly increase temperature over time. The block diagram for the instrumentation of TGA is illustrated below:
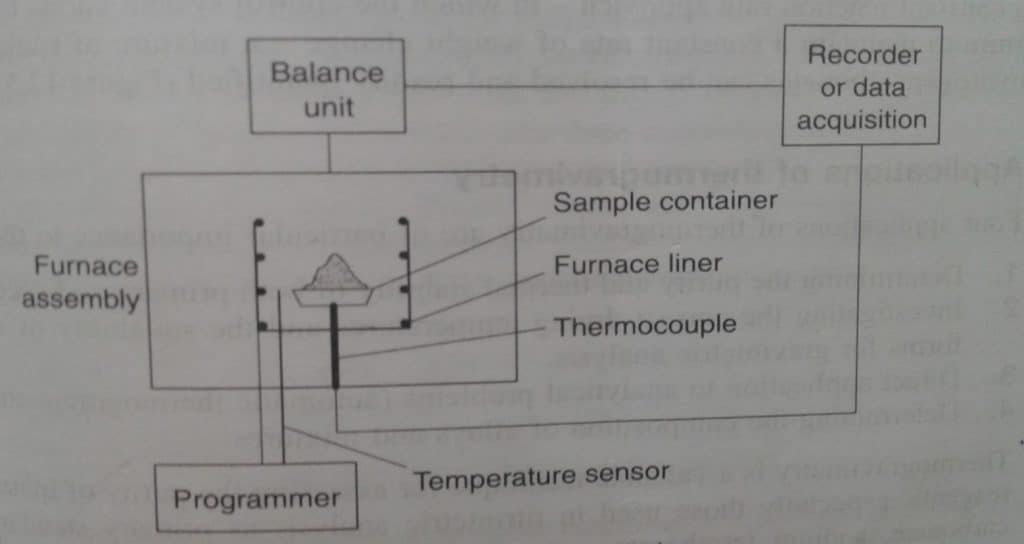
The major components of the thermogravimetric analyzer (thermobalance) are:
- Balance
- Furnace assembly
- Sample container, temperature sensor, furnace liner, thermocouple, etc.
- Recorder and display
- A purge gas system for providing an inert atmosphere.
The balance and the furnace assembly are the two key components of thermobalance. A shallow platinum crucible (also known as a sample container) is used to hold the samples and is connected to an automatic recording microbalance. In thermogravimetry, null point balances are the most often used form of the balance system. The null point approach causes the balance beam to move away from its normal position whenever there is a change in weight. Hence, a sensor detects this deviation and initiates a force that will restore the balance to the null position. This restoring force is proportional to the change in weight.
Some of the major requirements for the thermobalance are:
- Should be able to monitor the change in weight of the sample as a function of temperature.
- The rate of heating should be linear.
- The sample holder should be in the furnace’s hot zone, which should have a constant temperature. The balance mechanism needs to be shielded from corrosive gases and the furnace.
- Accurate measurement of the temperature of the sample.
Application of thermogravimetric analysis
Some of the major applications of TGA are:
- TGA is used for the thermal characterization of polymers.
- Determination of the composition of alloys and mixtures.
- Determining the purity and thermal stability of both primary and secondary standards.
- It is employed to investigate the moisture content of several inorganic and organic components, industrial raw materials, foods, medications, etc.
- Determining the ideal drying temperatures and the appropriateness of different weighing methods for gravimetric analysis.
- Used for corrosion studies.
- Also applicable to the study of kinetic of isothermal reactions.
Advantages of TGA
- Rapid process
- Short cooling time
- High accuracy of the balance
- A fast heating rate with good resolution can be maintained.
Limitations of TGA
- For both qualitative and quantitative analysis, only solid samples are allowed.
- TGA does not exhibit any chemical or physical alterations that do not result in a change in mass/weight when heated.
- Change in temperature as a result of being sensitive to heating rate and sample masses.
- Limited to samples that undergo weight change.
Differential Scanning Calorimetry and Thermogravimetric Analysis
Differential scanning calorimetry and thermogravimetric analysis are among the types of thermal analysis. Thermogravimetric analysis is used to characterize materials by measuring their change in mass as a function of temperature.
In differential scanning calorimetry, the difference in energy inputs into a substance and reference material is measured as a function of temperature while the substance and reference are subjected to a controlled temperature program.
TGA Thermogravimetric analysis Video
References
- Vogel’s Textbook of Quantitative Chemical Analysis, 6th Edition, 2008.
- H. H. Willard, L. L. Merritt, J. R. Dean, and F. A. Settle, Instrumental Methods of Analysis (7th Edition), CBS Publishers and Distributions, India, 1986.
- D.A. Skoog, F.J. Holler and T.A. Nieman, Principle of Instrumental Analysis, 5th Edition.






