Table of Contents
ToggleFlame photometry, also known as Flame Emission Spectroscopy is a branch of spectroscopy that is concerned with the emission of characteristic radiation in flames by individual elements and the correlation of the emission intensity with the concentration of these elements. It is based on the intensity of light that is emitted when metal is placed in a flame, with the intensity of wavelength providing information about the concentration of element and the color of wavelength giving information about the elements present in the sample.
Flame photometry is most applicable to determine the concentration of alkali and alkaline earth metals like sodium, potassium, lithium, calcium, barium, and so on.
Flame Photometry Principle
The neutral atom spectra are of primary interest in flame photometry; spectra from the ions may be formed at high temperatures and at high atom concentrations in the flame.
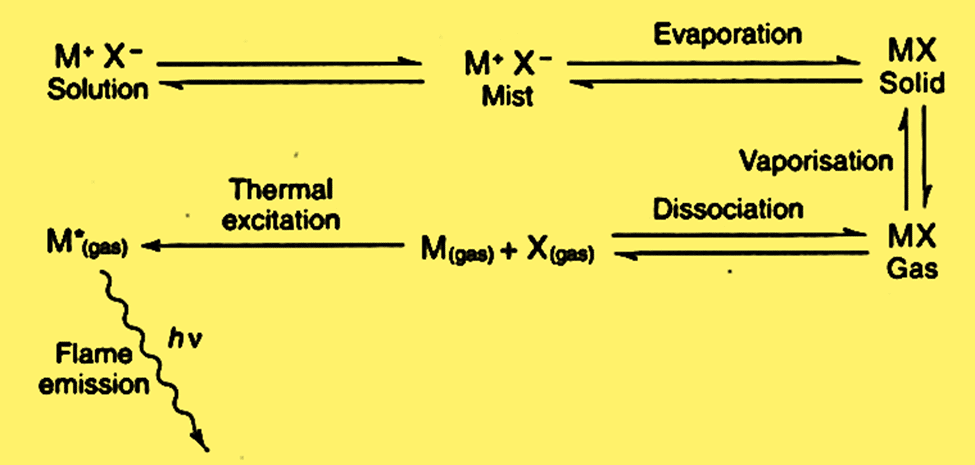
The following events happen in rapid succession when a solution containing a sufficient compound of the metal under investigation is introduced into a flame.
- Solvent evaporation leaving behind a solid residue
- Vaporization of the solid with dissociation into its individual atoms, each of which will initially be in the ground state. However, some atoms may be driven to higher energy levels by the thermal energy of the flame and eventually reach a state where they can emit energy.
The Boltzmann equation gives the relation between the ground state and the excited state.
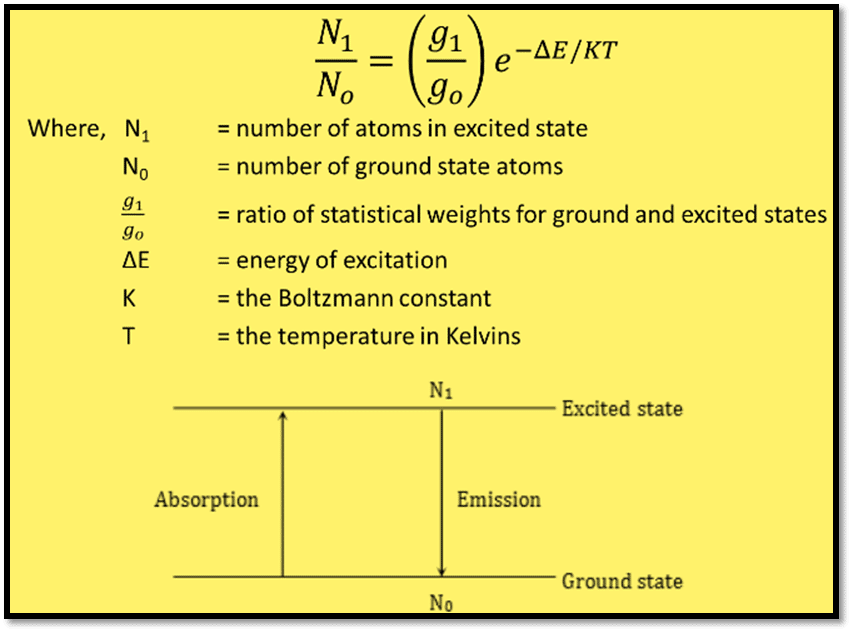
Under steady and controlled conditions, the number of emitting atoms and the concentration of the substance of interest in the sample is precisely proportional to the light intensity of the characteristic wavelength emitted by each atom.
Different colors of lights are observed when the metals are heated.
| Element | Emitted wavelength (nm) | Colour of flame |
| Lithium | 670 | Red |
| Sodium | 589 | Yellow |
| Potassium | 766 | Violet |
| Calcium | 662 | Orange |
| Barium | 554 | Lime green |
Instrumentation of flame photometry
The main components involved in flame photometry instrumentation are Flame, Burner, Mirrors, Nebulizer, Monochromator or filter, Detector, Amplifier, and Readout. The instrumentation can be illustrated as below:
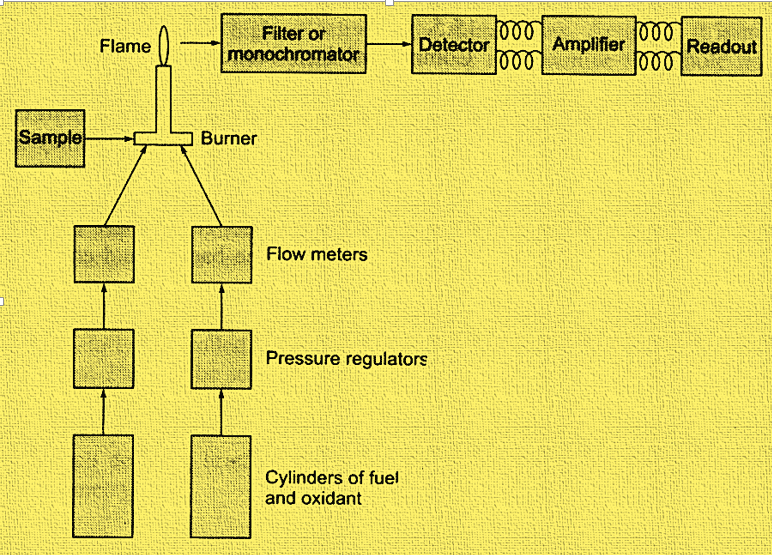
1. Nebulizer – burner system:
The production of gaseous atoms is the primary purpose of the nebulizer-burner system. The flame source is made up of a variety of parts, including a pressure regulator, a flow meter for fuel and oxidant, an atomizer, and a burner. Acetylene, hydrogen, and propane are among the most used gases. Likewise, air, oxygen, and nitrous oxide are the common oxidants employed.
The flame used in the flame photometer must be able to (i) evaporate the liquid droplets from the sample solution, resulting in the formation of solid residue. (ii) should decompose solid compound resulting the formation of gaseous atom. (iii) must have the capability to excite the atoms and cause them to emit radiant energy.
2. Monochromators / Filters
Flame emission lines or bands are isolated using monochromators, which allow them to pass through.
3. Detector
The main function of the detector is to detect the emitted light and measure the intensity of radiation emitted by flame via the conversion of emitted light into an electrical signal
4. Amplifier
It amplifies the signal from the detector which is received by the read-out.
5. Readout
It reads the data and gives the information regarding the metals, quantitatively.
Interference in flame photometry
Because the sample contains other materials, the radiation intensity might not precisely reflect the concentration of the sample, and hence results in interference. Some of the common interferences observed in flame photometry are:
- Spectral interferences
- Chemical interferences
- Ionization interferences
- Cation – anion interferences
- Cation – cation interferences
- Oxide formation interferences
Application of flame photometry
Flame Emission Spectroscopy is widely used in clinical chemistry, food, and agriculture, environmental analysis, biomedical research and pharmaceuticals industries, and so on. Some of the major applications are:
- Determination of calcium and magnesium in cement.
- Estimation of sodium, and potassium in the glass.
- Lead estimation in petrol.
- Applicable for the analysis of water via determination of Na, K, and Ca.
- To measure the amount of calcium and potassium in the soil, plant materials, food, and beverages.
- To determine the amount of metals, such as sodium, potassium, calcium, and lithium, in a sample of serum, urine, and other bodily fluids.
Advantages of Flame Photometry
- Rapid, inexpensive, and more convenient.
- A simple method for quantitative analysis based on flame analysis.
- Selective, and sensitive technique up to a wide range (even parts per million to parts per billion range).
Limitations of Flame Photometry
- Not applicable for the direct determination of metals.
- Liquid samples only can be analyzed.
- Not applicable for Non-radiating elements such as C, H, and halides since these elements cannot be detected by a flame photometer.
- It fails to provide information about the molecular form of metals present in the analyzed sample.
Difference between flame photometry and atomic absorption spectroscopy
| Flame Photometry (Flame emission spectroscopy) | Atomic absorption spectroscopy (AAS) |
| It involves emission phenomena. | It involves absorption phenomena. |
| Radiation emitted by excited atoms is related to the concentration. | Radiation absorbed during excitation is related to concentration. |
| Mainly used for the analysis of alkali and alkaline earth metals. | Elements like Al, Ag, Au, Cu, Cd, Hg, As, etc. are analyzed by AAS. |
| Greater spectral interferences. | Lesser in spectral interferences. |
| Lesser sensitivity | Higher sensitivity for most elements. |
Flame photometry Video
References
- Vogel’s Textbook of Quantitative Chemical Analysis, 6th Edition, 2008.
- H. H. Willard, L. L. Merritt, J. R. Dean, and F. A. Settle, Instrumental Methods of Analysis (7th Edition), CBS Publishers and Distributions, India, 1986.
- D.A. Skoog, F.J. Holler and T.A. Nieman, Principle of Instrumental Analysis, 5th Edition.






