Table of Contents
ToggleDifferential thermal analysis, also known as DTA is a type of thermal analysis that involves the measurement of the temperature difference between a substance and a reference material as a function of temperature while the substance and reference are subjected to a controlled temperature program.
Thermal methods of analysis are techniques that assess changes in a substance’s physical and/or chemical properties as a function of temperature. It involves changes in weight, energy, and so on. The physical changes involve adsorption, desorption, sublimation, evaporation, and vaporization while the chemical changes involve oxidation, reduction, degradation, chemisorption, etc. DTA peak results from both physical changes and chemical reactions induced by temperature changes in the sample.
Differential thermal analysis principle
In the DTA (Differential thermal analysis) method, a substance’s temperature difference from reference material (such as alumina or glass) is measured as a function of temperature while the substance and reference materials are placed through a controlled temperature program. The temperature program typically entails heating the sample and reference material so that the sample Ts temperature rises linearly over time.
The differential thermogram is created by plotting the temperature difference (ΔT) between the sample temperature (Ts) and the reference temperature (Tr) against the sample temperature. The graph of ΔT as a function of temperature provides a clue as to whether the sample under study has gained or lost energy. The reaction is exothermic if ΔT is negative, endothermic if ΔT is positive, and there is no physical or chemical change in the sample if ΔT is zero.
The shape and size of the peaks provide enough information about the nature of the test sample.
- Sharp endothermic peaks indicate a change in the fusion process or crystallinity.
- A broad endothermic peak appears due to dehydration reactions.
- While chemical reactions, especially those of an oxidative character, are primarily exothermic, physical changes typically produce endothermic curves.
Differential thermal analysis curves help in the qualitative identification of the substances via the shape, number, or position of various endothermic and exothermic features.
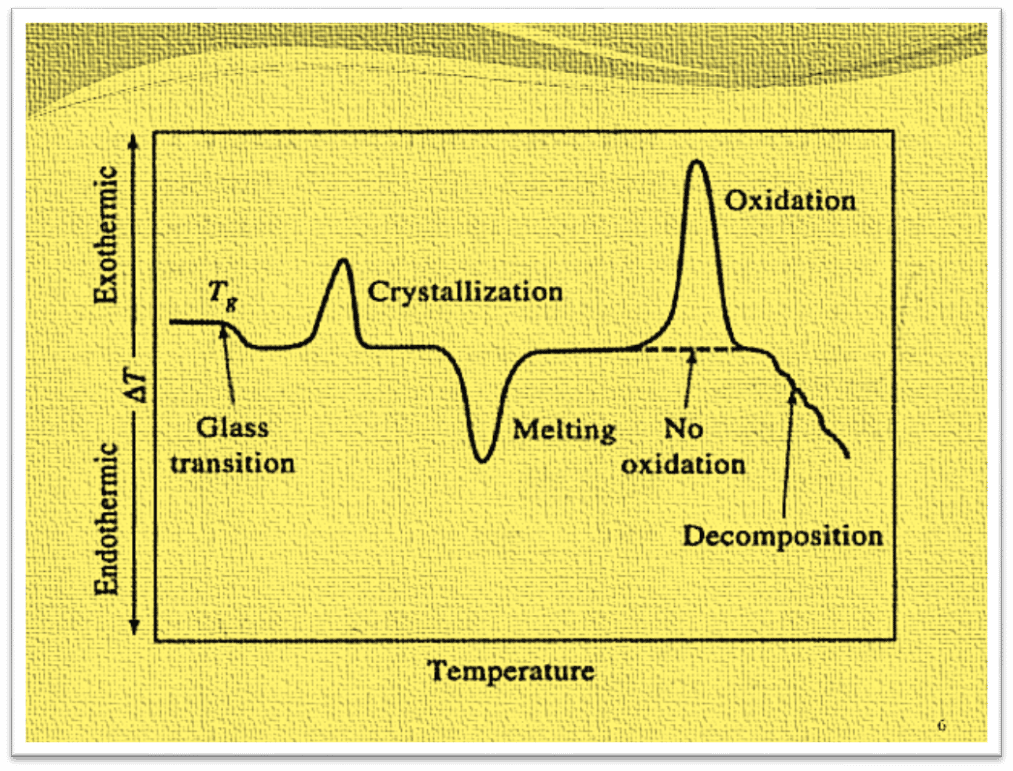
As shown in the plot, the initial fall in ΔT is due to a phenomenon known as glass transition which occurs when numerous polymers are heated. The glass transition, a phenomenon first noticed when various polymers are heated, is the cause of the initial fall in T. The Tg is the typical temperature where rubber-like or flexible properties emerge in glassy amorphous polymers i.e. glassy amorphous polymers become elastic or rubber-like. No heat is absorbed or evolved during such a transition, hence there is no change in enthalpy, or ΔH = 0. Since there is no enthalpy change during this transition, no peaks are formed.
The two peaks so-called maxima and minima appeared in the plot. The minima, denoted melting, is the outcome of an endothermic process in which heat is absorbed by the analyte. The two maxima are the result of exothermic processes in which heat is generated from the sample, causing its temperature to rise. Many amorphous polymers start to crystallize into microcrystals at a certain temperature, producing heat in the process.
The initial exothermic peak results from crystal growth. Since more crystals must form and grow in this situation, the area underneath such a peak increases larger as the heating rate decreases. The endothermic second peak is caused by the melting of the microcrystals created during the initial exothermic process. Exothermic in nature, the third peak may only occur when the heating is done in presence of air or oxygen which is due to the exothermic oxidation of the polymer.
The final negative change in ΔT results from the endothermic decomposition of the polymer to produce a variety of products.
Differential thermal analysis instrumentation
Two types of DTA instrumentations are available; classical instrumentation, and calorimetric DTA instrument. The classical instrumentation is shown below:
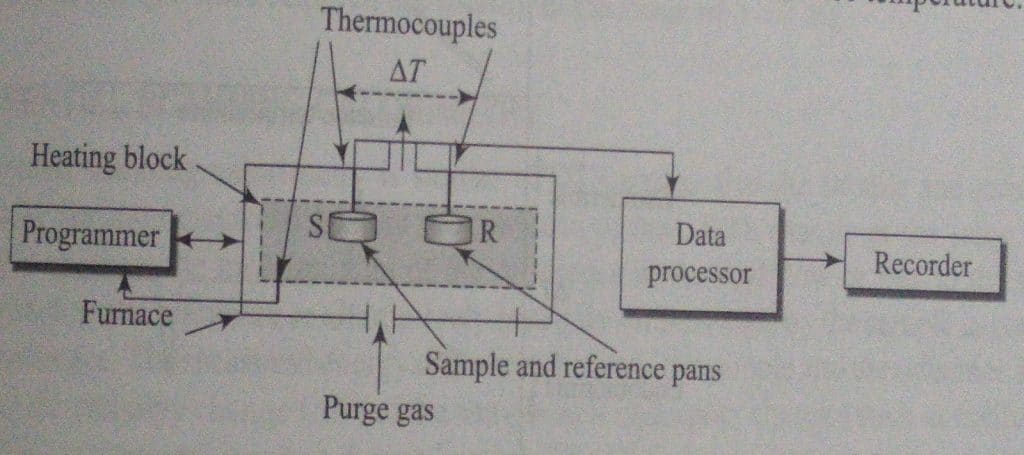
DNA instrumentation consists of the following components:
- Furnace: The main function of furnance is to supply heat to both the sample and reference at the same rate.
- Sample holder: It is used to hold both the reference materials and the sample. The materials utilized to create the sample holder are both metallic (nickel, stainless steel, platinum and its alloys) and non-metallic (glass, vitreous silica).
- Thermocouples: It permits the measurement of the the temperature of the furnace and that of the reference and sample.
- Programmer: The furnance programmer is used to increase the temperature of the furnace at a steady rate.
- Furnace temperature control system: a device that enables pure gas to keep the furnace and sample holder’s environment at an appropriate level.
- DTA processor and recorder
In a classical DTA device, alumina, silicon carbide, or glass beads are used as the sample and reference materials, respectively, and are deposited in two identical crucible-like fissures on a single heating block. The heating block is then placed in a furnace and heated uniformly. By placing individual thermocouples in contact with the samples, the temperatures of the sample (TS) and the reference (TR) are each measured separately. The temperature of the furnace is controlled using a third thermocouple. The DTA thermogram can be developed by plotting the difference (ΔT) between the test sample and the reference material (ΔT = TR – TS) as a function of furnace temperature.
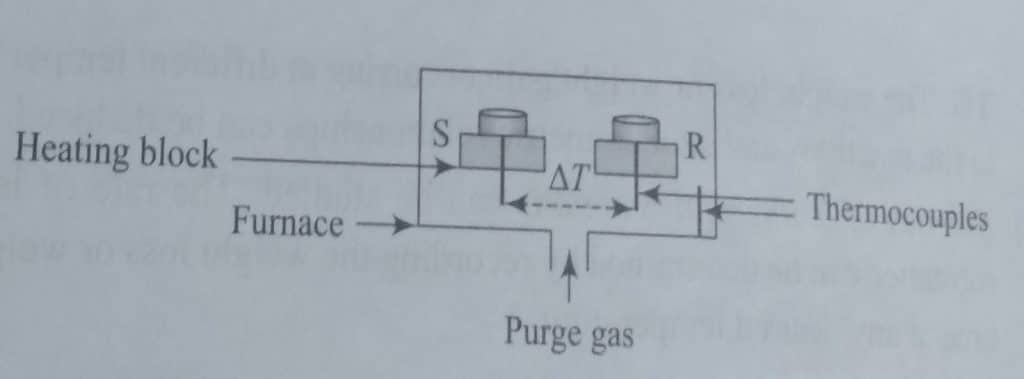
The calorimetric DTA instrument is the second type of DTA instrument, and it uses two pans stored on different heating blocks for the sample and reference materials. Instead of being in contact with the samples, the thermocouples are placed in contact with the pans (or heating blocks), and a third thermocouple measures the furnace temperature as in the classical instrument.
Applications of Differential thermal analysis
DTA have a wide range of applications in pharma industry, food industry, cometic industry, polymer industry, ceramic industry, and so on.
Some of the major applications of DFT are:
- Used for determination of heat of reaction, specific heat and energy change occurring during melting etc.
- For the generation of phase diagrams and the study of phase transition.
- For the accurate dtermination of the melting, boiling and decomposition points of organic compounds.
- Investigation of product purity in pharmaceutical industry.
- This method can be used to check the quality control of a variety of substances, including cement, soil, glass, and drug samples.
Advantages and Disadvantages of differential thermal analysis
Some of the major advantages and disadvantages of differential thermal analysis are:
Advantages of DTA
- Highly sensitive instruments
- Applicable at very high temperature
- Accurate determination of transition temperature
Disadvanatges of DTA
- Fails to determine small change in ΔT
- Less accurate determination of ΔT.
Differential Thermal Analysis Video
References
- Vogel’s Textbook of Quantitative Chemical Analysis, 6th Edition, 2008.
- H. H. Willard, L. L. Merritt, J. R. Dean, and F. A. Settle, Instrumental Methods of Analysis (7th Edition), CBS Publishers and Distributions, India, 1986.






