Table of Contents
ToggleGravimetric analysis is a quantitative approach in analytical chemistry that is based on determining the quantity of analyte based on the mass of the solid. Generally, analyte is physically separated from the components even from the solution. Some of the common methods of separating the analyte from other interferences include precipitation, electrolysis, solvent extraction, chromatography, and so on.
Principle of Gravimetric Analysis
A general principle of gravimetric method of analysis is based on a chemical reaction between analyte and reagent.

The analyte (A) of molecules ‘a’ react with the reagent (R) of molecule ‘r’. After drying, the product formed by igniting AaRr can either be weighed or ignited to create another compound of known chemical components.
For example, the precipitation of calcium oxalate and ignition of it into CaO can be used to determine calcium using gravimetry.
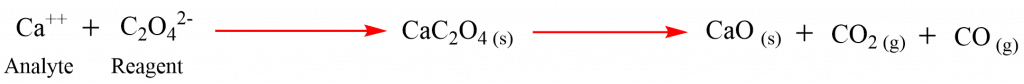
Note: The reagent is always needed in excess amounts to decrease the solubility of the precipitate.
Types of Gravimetric Analysis
Based on the separation of analyte, gravimetric analysis is of the following 4 types:
- Precipitation Gravimetry: The separation of one or more components of a solution by incorporating them into a solid is accomplished by a precipitation reaction.
- Voltalization Gravimetry: This involves either heating the solution or chemically decomposing it in order to separate the target ion from the sample’s solution.
- Electrogravimetry: Used to separate and analyze the ions in a substance like a metal.
- Thermogravimetry: Based on changes in chemical and physical properties that have been observed over a period of time at various temperatures, the mass of the sample is determined.
Requirements of Gravimetric Analysis
- The amount of analyte that remains unprecipitated should be less than 0.1 mg or less, demonstrating that the separation of the analyte should be sufficiently complete.
- The substances being weighed must be precise and pure, or extremely close to being pure, in order to eliminate error.
To obtain pure precipitate, the second requirement is usually a challenge for the chemist. Therefore, one needs to be familiar with precipitate formation and its properties in order to perform gravimetric analysis.
Gravimetric Analysis Steps
- Preparation of solution of known sample
- Precipitation
- Filtration and washing of precipitate
- Drying and Ignition
- Heating to constant weight
Advantages of Gravimetric Analysis
- It is one of the most accurate methods for chemical analysis when performed correctly.
- It is an absolute method that only uses direct measurement and requires no calibration.
- Used for the determination of almost all cations and anions.
- Also applicable for neutral species such as CO2, H2O, SO2, etc.
- Employed for the estimation of a large variety of organic compounds like salicylate in drugs, nicotine in pesticides, and so on.
Disadvantages of Gravimetric Analysis
- It is only applicable for the analysis of a single element or a limited group of elements, at a time.
- This method frequently involves complicated steps, and even a slight error can have disastrous effects on the analysis.
- It is a time-consuming method since a large number of steps are involved.
Application of Gravimetric Analysis
Some of the common applications of Gravimetric analysis are:
- Quantification of inorganic and organic compounds
- Elemental analysis
- Uses in modern analytical chemistry
- High Sensitivity and accuracy of gravimetric method
Errors in Gravimetric analysis and Precautions
Errors in gravimetric analysis arise due to inaccurate weighing, incomplete and faulty precipitation, incomplete washing of precipitate, use of substandard reagent, ignition of precipitate at extremely high or low temperatures, and so on.
To avoid such errors, the following precaution must be taken for successful gravimetric analysis:
- Since gravimetric analysis is a lengthy and laborious process, all the steps must be completed with great patience.
- The precipitation should be carried out in a dilute solution and a hot condition because dilutions prevent co-precipitation, while precipitation is effective in a hot solution.
- The precipitant should be added slowly with constant stirring.
- Filter paper like Whatman no. 41/42 should be used for filtration.
- Heating and weighing of precipitate must be continued till constant weight is obtained.
Difference Between Gravimetric and Volumetric Analysis
| Gravimetric Analysis | Volumetric Analysis |
| Determination of the quantity of analyte based on its mass. | Determination of amount of analyte based on its volume. |
| Involves chemical reactions that can lead the desired substance to precipitate. | involves chemical processes that have the potential to change the sample’s color |
| Determination of lead in water is one example of it. | Estimation of Fe2+ by titrating against acidified KMnO4 is an example of this analysis. |
| The result is obtained in grams. | The result is obtained in milliliters. |
Gravimetric Analysis Video
FAQs
What is gravimetric analysis?
Gravimetric analysis is a quantitative determination of an analyte based on the mass of a solid.






