Table of Contents
TogglePrecipitation titration is such a titrimetric analysis that involves the formation of sparingly soluble compounds called precipitate. Precipitation refers to the formation of a solid in a solution, while the solid formed is called a precipitate. The cation comes from one solution, while the anion comes from another, making the precipitate itself ionic.
In this titration, the analyte and titrant combine to form an insoluble substance, and the process is repeated until all of the analytes have been consumed. The first drop of excess titrant will react with an indicator, changing color and indicating the end of the titration.
Moreover, precipitation Titration is used to determine the presence of metal ions and halogens.
Principle of Precipitation Titration
The basic principle behind precipitation titration is that the amount of precipitant or precipitating agent that is added must be equal to the substance that is being precipitated.
Characteristics of Precipitation Titration
- They are fast and have a known and reproducible stoichiometry.
- Depending on how much solubility product is present, they are complete or can be quantified (generally, a precipitation titration is thought to be completed when Kps 10-8).
- The equivalence point or titration endpoint, for this kind of titration, corresponds to the point at which the analyte under evaluation has completed precipitating.
Requirements of Precipitation Titration
- The precipitate must be practically insoluble.
- The precipitation should be rapid.
- The precipitate formation is stoichiometric.
- No Change in the oxidation state.
- The titration results should not be distorted appreciably by adsorption.
- It must be possible to detect the equivalence point during the titration.
Argentometric Titration
It is a form of precipitation titration that uses silver ions. In general words, titrations involving silver ions are called argentometric titration. The precipitation of silver salts occurs at rapid reaction rates. The main precipitation reaction in use involves silver and a variety of anions. A few of these anions are halides (Cl–, Br–, I– ), pseudohalides (HS–, CN–, SCN–), etc.
Types of Precipitation Titration
According to the endpoint detection method, there are following three methods of precipitation titration:
Mohr’s Method
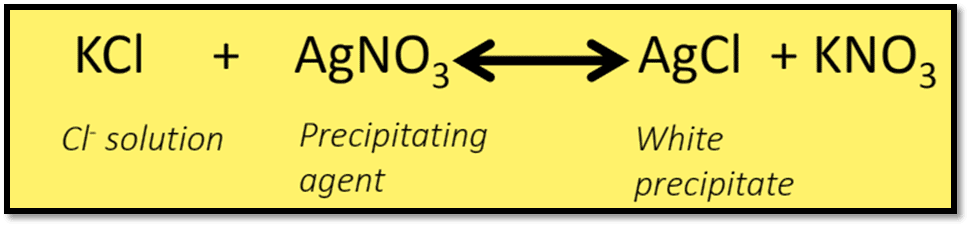
Titration of a halide, such as NaCl, with an AgNO3 solution while the presence of K2CrO4 is the basis of Mohr’s method. Due to the presence of CrO4— ions in the solution, the suspension’s color changes from yellow to reddish brown at the endpoint.
Formation of both ppt. AgCl and Ag2CrO4 occur. AgCl precipitates out first because of less solubility and a high concentration of Cl–. When all Cl– ion have been precipitated as AgCl, the next drop of AgNO3 form a brick-red color due to the formation of Ag2CrO4.
Volhard’s Method
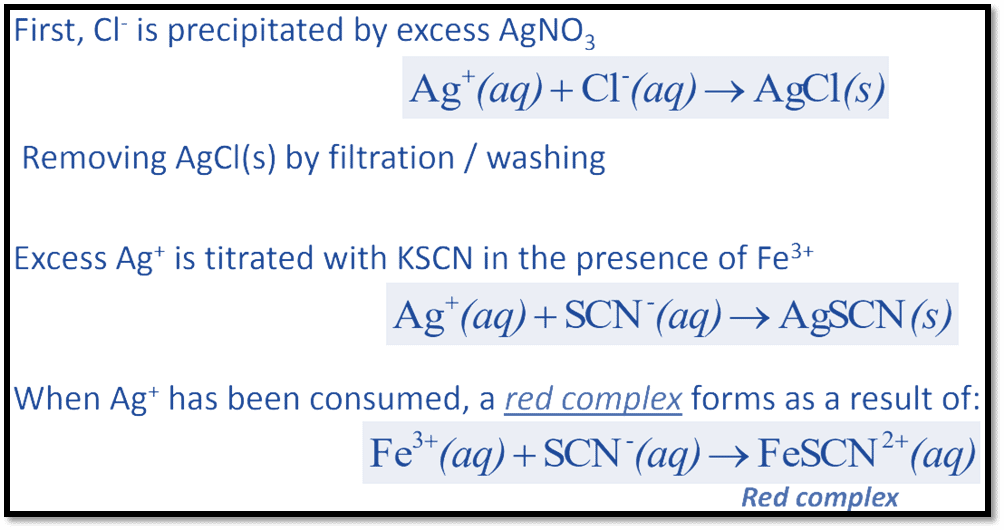
This method can be used to determine the presence of chlorides, bromides, and iodides in acidic solutions and involves a back titration with potassium thiocyanate. Any anion that reacts with silver to generate an insoluble salt is suitable for Volhard titration.
The chloride in the solution is changed into silver chloride in this type of reaction with the excess of silver nitrate. Potassium thiocyanate solution is once more used to treat the silver nitrate that remains after the reaction. A complex is created when the excess thiocyanate reacts with an indicator, which turns red when it interacts with the ferric ammonium sulfate indicator.
The whole process can be illustrated as:
Fajan’s Method
This technique developed by Kazimierz Fajans, an American chemist, employs the indicator absorption method. In this approach, dichlorofluorescein served as an indicator, and excess methyl chloride ions are absorbed on the surface of silver chloride. At the endpoint, the indicator adheres to the silver salt precipitate’s surface resulting in the indicator’s color changes as a result of the adsorption process.
Adsorption Indicators
When a colored organic compound is adsorbed on the surface of the precipitate, modification of the organic structure may occur, and the color may be greatly changed and become more intense. This phenomenon can be used to detect the endpoint of precipitation titration of silver salts. The organic compound thus employed is referred to as an adsorption indicator. In general words, adsorption indicators are organic compounds that are weakly acidic and colored. Eosin (tetrabromo fluorescein), Fluorescein, Tartrazine, Chloroflourescein, etc. are some of the common adsorption indicators.
Mechanism of adsorption
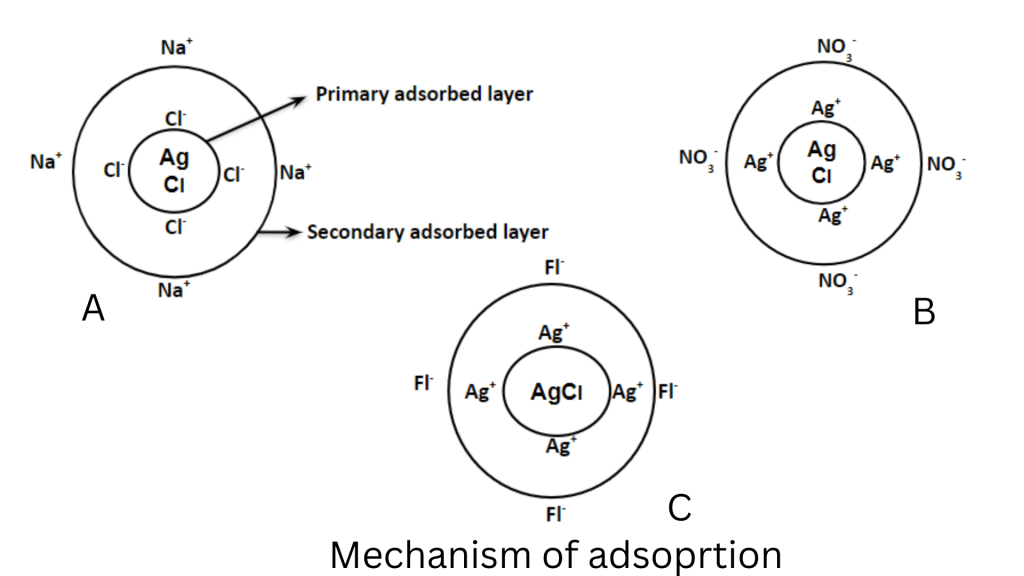
The theory behind how these indicators work is based on the characteristics of colloids (0.1 µm – 1 nm). Let us consider titration between NaCl and AgNO3.
Because a ppt. has a tendency to adsorb its own ion, when a chloride solution is titrated with solution AgNO3, the ppt. AgCl adsorbs a chloride ion. This could be referred to as the primary adsorbed layer. Opposingly charged ions in the solution are kept around it by a process called secondary adsorption.
When the endpoint is achieved, the excess Ag+ ions become primarily adsorbed, however, the NO3– ion is kept by secondary adsorption.
If an indicator, such as fluorescein, is present in the solution, the negative fluorescence ion is quickly absorbed in the second adsorption layer, taking the place of NO3–. Because the indicator has stronger adsorption than NO3–. With the first excess of Ag+ ion, a pink complex of Ag+ and fluorescein forms.
The whole mechanism can be illustrated as:
Condition for choice of suitable adsorption Indicator
- The precipitate should separate as far as possible in the colloidal condition.
- The indicator ion must have a charge opposite to that of the precipitate ion.
- The solution shouldn’t be excessively diluted. If so, there won’t be much precipitation, and the color changes won’t be noticeable.
- Not before the precipitate has fully formed, but only immediately after the equivalence point, the indicator should be strongly absorbed.
Application of Precipitation Titration
- It is employed to identify the presence of halide ions in solutions.
- It is applied to determine the amount of salt in food, water, and beverages.
- Used to analyze numerous drugs like KCl infusion, carbromal, NaCl infusion, and so on.
- The concentration of the anion in the analyte is determined using this titration.
- It also serves as a secondary analytical technique to validate primary analytical techniques.
Limitations of Precipitation Titration
- Co-precipitation may occur.
- Applying the precipitation titration method, only a few halide ions can be titrated.
- Determining the endpoint is really challenging.






