Table of Contents
ToggleIonic compounds include salts, oxides, hydroxides, sulfides, and the majority of inorganic compounds. Ionic solids are held together by the electrostatic attraction between the positive and negative ions. There will be repulsion if ions of the same charge are adjacent, and the attraction will occur when positive ions are surrounded by negative ions, and vice versa.
Classification of Ionic Structures
It is convenient to divide ionic compounds into groups AX, AX2, AX3 depending on the relative numbers of positive and negative ions.
Ionic Compound of the Type AX
Three structural arrangements commonly found are zinc sulfide, sodium chloride, and cesium chloride structures.
Structures of zinc sulfide
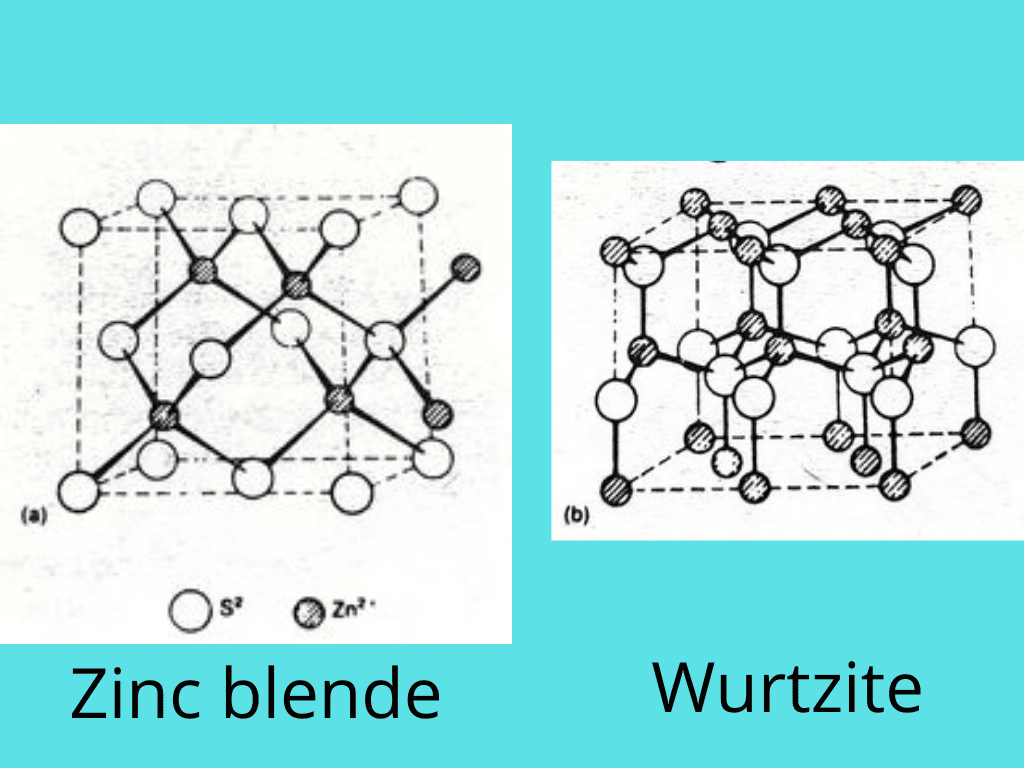
In zinc sulfide, ZnS, the radius ratio of 0.40 suggests a tetrahedral arrangement. Each Zn2+ ion is tetrahedrally surrounded by four S2– ions, and each S2– ion is tetrahedrally surrounded by four Zn2+ ions. The coordinate number of both is 4, so this is called a 4:4 arrangement. Two different forms of zinc sulfide exist: zinc blende and Wurtzite. Both are 4:4 structures.
Sodium chloride structure
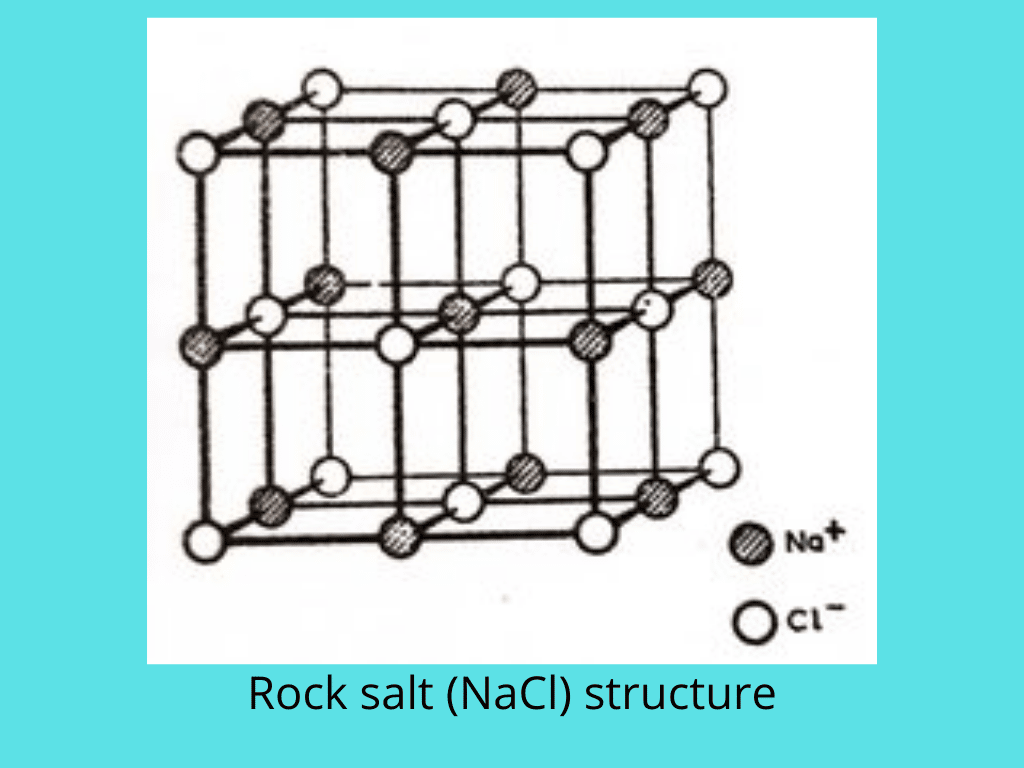
For sodium chloride, NaCl, the radius ratio is 0.52, and this suggests an octahedral arrangement. Each Na+ ion is surrounded by six Cl– ions at the corners of a regular octahedron, and similarly, each Cl– ion is surrounded by six Na+ ions. The coordination is thus 6:6. The structure may be regarded as a cubic close-packed array of Cl– ions, with Na+ ions occupying all the octahedral holes.
Cesium chloride structures
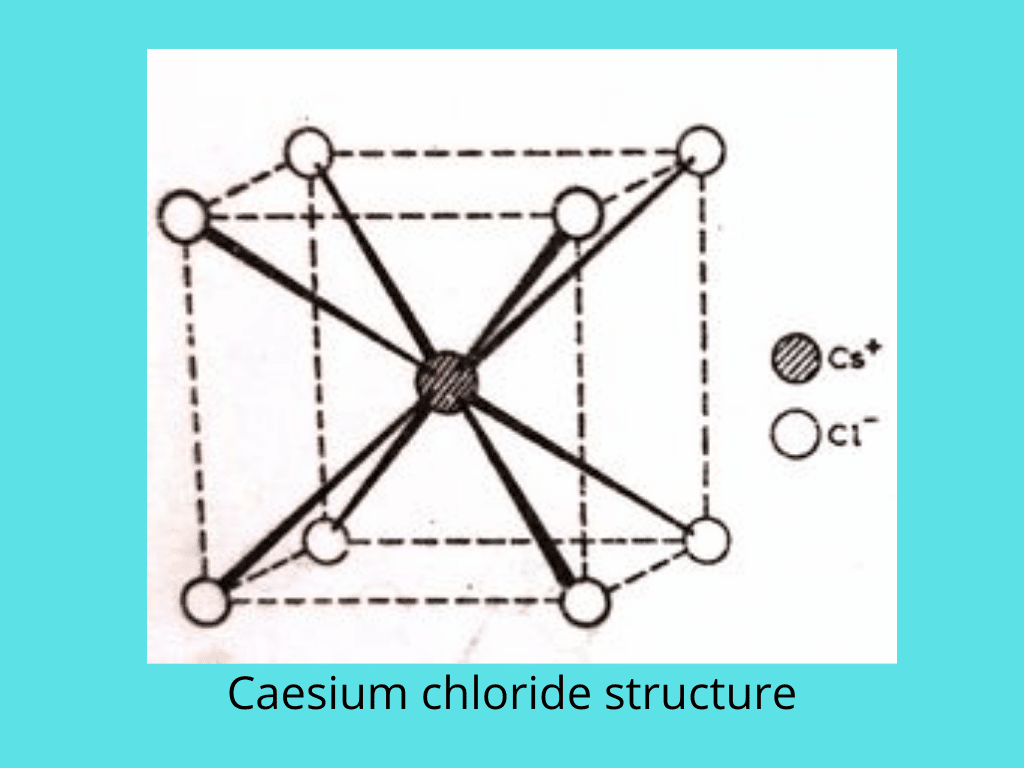
In cesium chloride, CsCl, the radius ratio is 0.93. This indicates a body-centered cubic type of arrangement, where each Cs+ ion is surrounded by eight Cl– ions, and vice versa. The coordination is therefore 8:8. This structure is not tightly packed and is not strictly body-centered cubic.
Ionic Compound of the Type AX2
The two most common structures are fluorite, CaF2, and rutile, TiO2, and many difluorides and dioxides have one of these structures.
Calcium fluoride structures
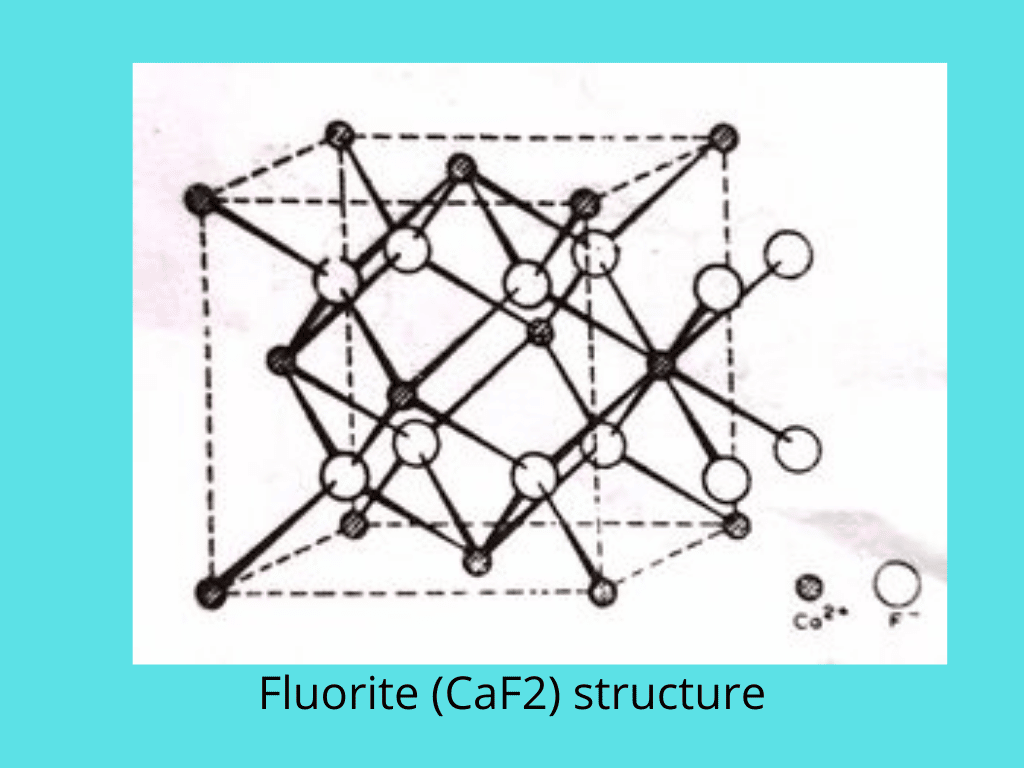
In fluorite, each Ca2+ ion is surrounded by eight F– ions, giving a body-centered cubic arrangement of F– round Ca2+. Since there are twice as many F– ions as Ca2+ ions, the coordination number of both ions is different, and four Ca2+ ions are tetrahedrally arranged around each F– ion. The coordination numbers are therefore 8 and 4, so this is called an 8:4 arrangement.
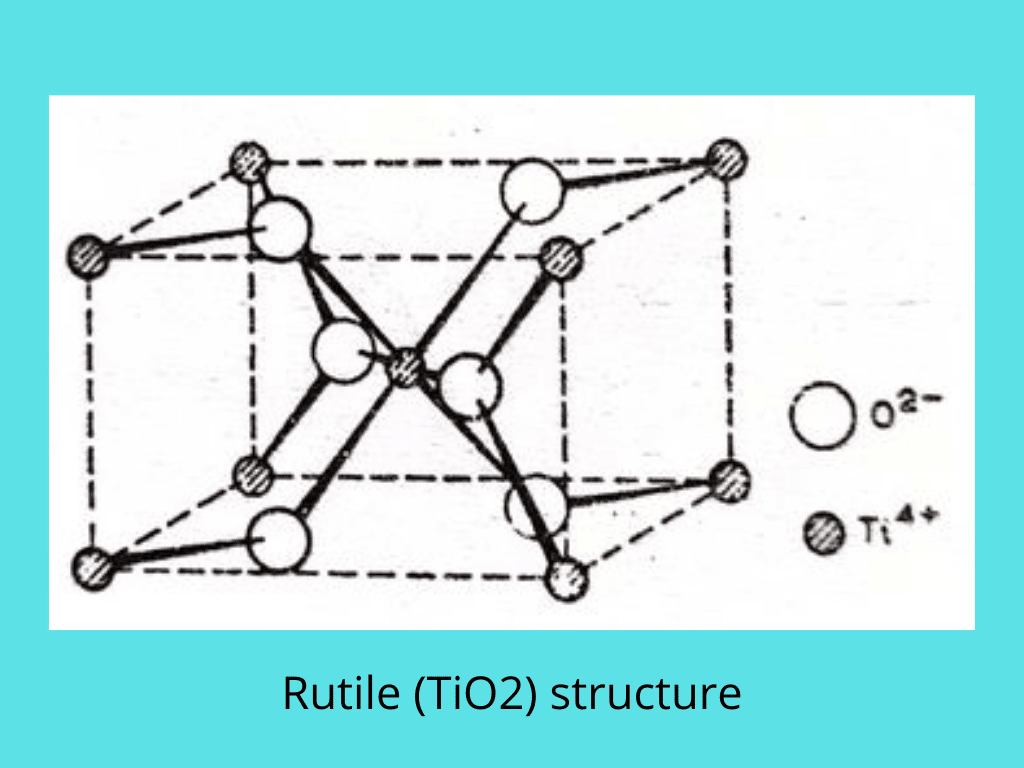
Rutile structure
TiO2 exists in three forms called anatase, brookite, and rutile. The rutile structures are found in many crystals where the radius ratio is between 0.41 and 0.73. This suggests a coordination number for one ion, and from the formula, it follows that the coordination number of the other ion must be 3. This is a 6:3 structure. Each Ti4+ is octahedrally surrounded by six O2-ions, and each O2- ion has three Ti4+ ions round it in a plane triangular arrangement.
References
- Atkins, P. (2010). Shriver & Atkins’ Inorganic Chemistry (later Edition). Oxford University Press.
- Lee, J. D. (2008). Concise Inorganic Chemistry: Fifth Edition by J.D. Lee (Fifth edition). Oxford University Press.






