Table of Contents
ToggleComplexometric titration is such a volumetric analysis that involves the formation of a soluble but slightly dissociated complex or complex ion. The formation of the colored complex determines the endpoint of the titration. Metal ions in solutions are estimated by complexometric titration. This is the titration between the metal ion and complexing agent in presence of a suitable indicator. The indicator is also a complexing agent but it should form a less stable complex with metal ions and should impart different distinct colors in complexation and decomplexation. Some examples of complexometric indicators include calcein, Eriochrome Black T, curcumin, hematoxylin, fast sulphon black, etc.
The reaction quickly reaches equilibrium when each drop of titrant is supplied. There wouldn’t be any possibility of anything interfering. A complexometric titration can be used to locate the equivalent point with extreme accuracy.
EDTA is the most commonly used chelating agent in complexometric titrations. These titrations are very sensitive to pH and metal ion indicators are used to detect the endpoint. Most of the EDTA titrations belong to complexometric titrations.
EDTA Complexometric Titration
EDTA is a common organic reagent used in complexometric titration. It is a hexadentate chelating ligand (a powerful complexing agent) coordinated with metal ions through its two nitrogens and four carboxylic groups.
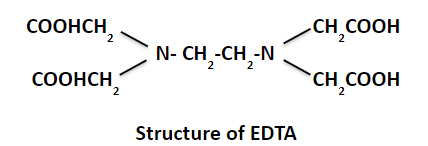
It is shortly written as H2Y2-. It reacts with most metals in a 1:1 ratio, one mole of H2Y2- reacts in all cases with one mole of the metal ion, and in each case, also, two moles of hydrogens are formed.
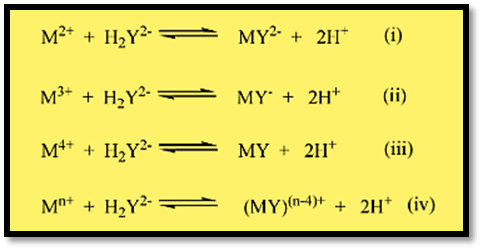
M-EDTA complexes with metal ions of charge number 2 are stable in alkaline (eg. pH 8-10: Ca2+, Sr2+, Ba2+, Mg 2+) or slightly acidic solution (pH 4-6; Pb2+, Zn2+, Co2+, Ni2+, Mn2+, Fe2+, Cd2+, Sn2+, with + 3 or + 4 (eg. pH 1-3: Zr+4, Hf+4, Th+4, Bi+3, Fe+3) may exist in solutions of much higher acidity.
EDTA complexes with metal as illustrated below:
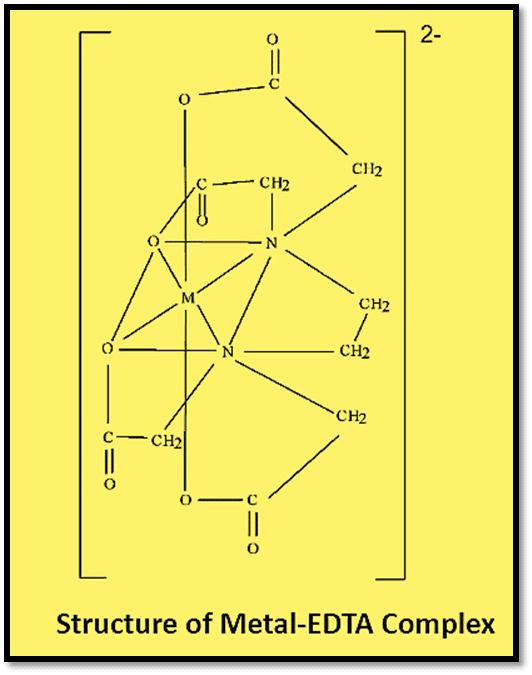
The stability of the complex is characterized by the stability constant (or formation constant), K:
Mn+ + Y4- ↔ (MY)(n-4)+
K = [(MY)(n-4)+ ] / [Mn+] [Y4-] (mostly expressed as logK)
Types of complexometric titration
- Direct Titrations: The solution containing metal ions is titrated directly with EDTA solution. It is like acid-base titration. The EDTA solution is added to the metal-containing sample and burette until the endpoint is reached in this titration standard. At the equivalence point, the concentration of the metal ion being determined decreases, which is determined by the change in color of the metal indicator. Metals such as copper, zinc, barium, mercury, aluminum, lead, chromium, bismuth, etc. can be identified using direct complexometric titration.
- Back Titration: Many metals can’t be titrated directly due to various reasons like a color not being distinguishable, lack of suitable indicator, a slow reaction between metal and EDTA, and so on. In such case, an excess of standard EDTA is added and the resulting solution is buffered to the desired pH. Then excess EDTA is back-titrated with a standard metal ion (eg. Mg2+ solution).
- Substitution or Replacement Titration: This type of titration is used for metal ions that do not react with a metal ion indicator or for the metal ions which form EDTA complexes that are more stable than those of other metals, such as Mg and Ca. In such a case, a solution containing the Mg-EDTA complex is added and the metal ion displaces the Mg from Mg-EDTA Complex. For example, titration of calcium: In the direct titration of calcium ions, solochrome black-T gives a poor endpoint, if Mg is present, it is displaced from its EDTA complex by calcium.
- Indirect Titration: When combined with metal cations, some anions generate a precipitate. With EDTA, these anions do not interact. Indirect titration with EDTA can be used to analyze them.
Metal ion indicators
Metal ion indicator is used for the precise determination of endpoint in complexometric titration. For visual detection of the endpoint, the metal ion indicator should satisfy the following criteria.
- To ensure that the color change happens as close to the equivalence point as feasible, the indicators must be extremely sensitive to metal ions.
- The color reaction should be specific or at least selective.
- Before the endpoint, when nearly all of the metal ion has complexed with EDTA, the color response must be such that the solution is intensely colored.
- The metal-indicator complex must possess sufficient stability, but the metal-indicator complex must be less stable than the metal-EDTA complex to ensure that, at the endpoint, EDTA removes metal ions from the metal-indicator complex. The change in equilibrium from the metal indicator complex to the metal-EDTA complex should be sharp and rapid.
- It should be simple to see the color contrast between the metal indicator complex and the free indicator.
- All these criteria must be fulfilled within the pH range at which titration is performed.
Examples of Metal ions indicators
Some examples of metal ions indicators are:
- Patton & Reeder’s Indicator (12-14 pH) (Ca)
- Murexide (10 – 11 pH) (Cu, Ni, Co, Ca)
- Solochrome black (10 pH) (Mg, Mn, Zn, Cd…etc)
- Xylenol orange (4-6 pH) (Ca, Pd, Ni…)
- Xylenol orange (1-2 pH) (Bi, Zn, Co…)
- Methyl thymol blue (0-2 pH) (Th, Zi, Hf, Zn, Co…etc)
Uses of EDTA in inorganic analysis
- Determination of water hardness.
- For the analysis of more than 40 cations like Al, Fe, Cu, Zn, Cr, Mg, Bi, Pb, rare earth, etc
- Determination of anions: eg. arsenate, chromate, fluoride, pyrophosphate, sulfate, etc.
- It can be used in the analysis of technological materials such as alloys, cement, coins, mineral oils, petrol, many ores, and rocks.
- EDTA as masking or sequestering reagent
Application of Complexometric Titration
- The hardness of water is estimated by complexometric titration.
- To determine the metal content in medicines, it is commonly employed in the pharmaceutical industry.
- Numerous cosmetic products contain titanium dioxide. By using complexometric titration, this can be analyzed.
- Analysis of urine samples.
- It has many applications in analytical chemistry.






