Table of Contents
ToggleA Danish chemist, Johan Kjeldahl invented the Kjeldahl method in 1883. This approach was created especially for figuring out how much nitrogen is in both organic and inorganic substances. The Kjeldahl method has been employed for determining nitrogen in a variety of organic and inorganic materials like foods and beverages, meat, feeds, cereals, and forage content in protein. Additionally, the Kjeldahl method is utilized in order to determine nitrogen in samples of soil, wastewater, and other things.
Kjeldahl method procedure
Kjeldahl nitrogen method is a recognized technique that is covered in the “main three procedures.”
1. Digestion:
The given or collected organic sample is first treated with a strong acid solution, primarily H2SO4. At a very high temperature, the solution is brought to a boil. The sample is broken down by the acid solution to create ammonium sulphate solution.
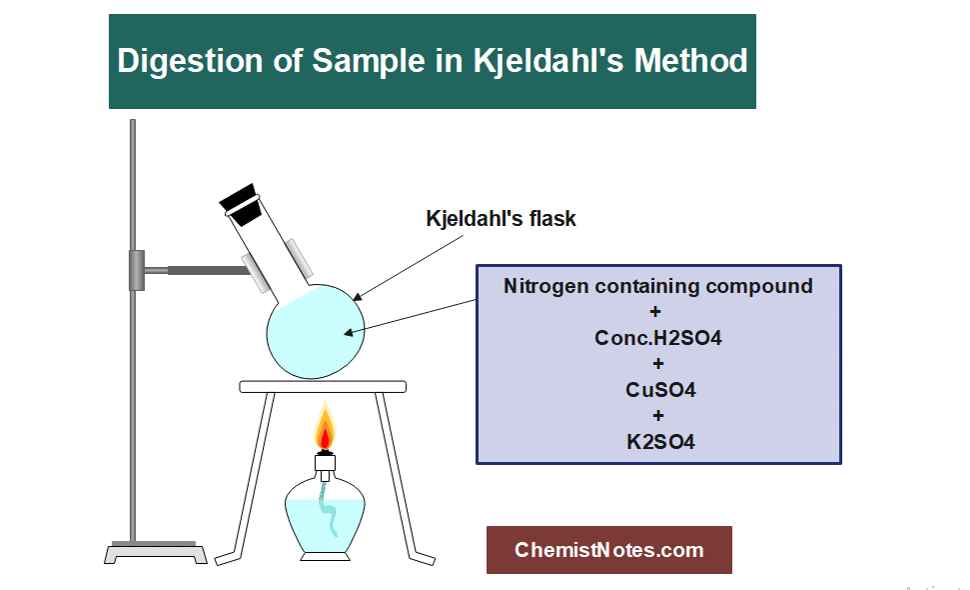
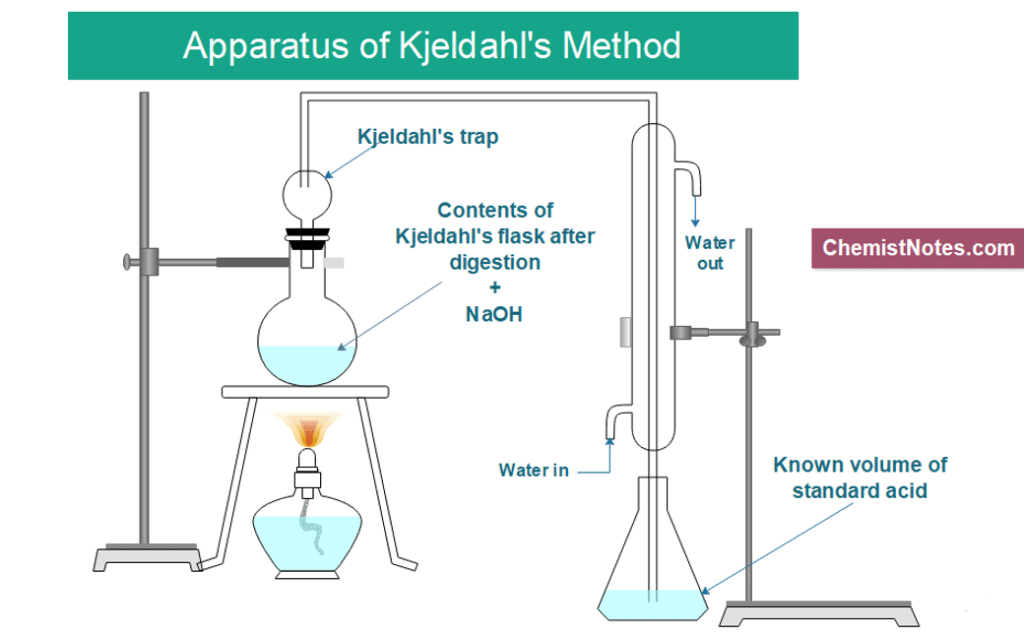
2. Distillation:
The specific procedure combines boiling and condensation. The ammonium sulfate solution is added to the created solution in excess to produce NH3 gas.
3. Titration:
The unreacted HCl in the solution is back-titrated with a standard NaOH in the final phase of the Kjeldahl method, and the concentration of HCl that has been utilized to neutralize the ammonia is then computed. However, in addition to HCl, the solution also contains the protonated form of NH3, an acid that can be titrated with NaOH.
Kjeldahl method reaction
1. Digestion
Organic (C, H, N) + H2SO4 → digest Cu2+ + (NH4)2SO4
2. Distillation:
(NH4)2SO4 + 2NaOH → Na2SO4 + 2H2O + 2NH3
And, NH3 + HCl → NH₄Cl
3. Titration:
B(OH)2 + H2O + Na2CO3 → NaHCO3 + CO2 + H2O
Kjeldahl method formula
NH3 has an equivalent mass of 17g/eq. And one equivalent weight of NH3 contains 14 grams of nitrogen. Consequently, the following formula can be used to calculate the nitrogen percentage:
Nitrogen estimation using the Kjeldahl technique equals 1.4V× NW, where W equals the sample’s weight (in grams).
V = Acid used in titration (in ml)
N = normality of standard acid
Working of Kjeldahl method
The procedure entails basically converting all of the nitrogen in a weighed sample into ammonium sulphate by digestion with sulfuric acid, alkalinizing the solution, and determining the ammonia that results by distilling it into a measured volume of standard acid, the excess of which is determined by titration.
Kjeldahl method for determination of protein
Johann Kjeldahl, a brewer, created the Kjeldahl technique in 1883. Strong acids are used to break down food, which releases nitrogen that can be measured using an appropriate titration technique. The food’s nitrogen content is then used to determine how much protein is there.
Sensitivity of Kjeldahl method
In its original form, the Kjeldahl method is not very sensitive. Other detection techniques have been used to measure NH4+ after mineralization and distillation, achieving improved sensitivity. These include potentiometric titration (>0.1 mg of nitrogen), zone capillary electrophoresis (1.5 g/ml of nitrogen), and ion chromatography (0.5 g/l).
Advantages of Kjeldahl method
- High precision, and reproducibility for the determination of protein in foods.
- Standard technique for evaluating all other techniques
Diasdvantages of Kjeldahl method
There are some drawbacks to the Kjeldahl digestion technique. Only ammonium and nitrogen that are bound to organic substances (proteins, amino acids, and nucleic acids) are measured by this approach. This method cannot be used to measure other nitrogen forms, such as nitrate and nitrite.
Published by: Pratiksha Chaudhary
FAQs
Which acid is used during the digestion process of Kjeldahl method?
Concentrated sulphuric acid is used during the digestion process of kjeldahl method.
Why is base used in this method?
To neutralise the excess sulfuric acid and release the ammonia.
Why is boric used during the Kjeldahl method?
The boric acid captures the ammonia gas, forming an ammonium-borate complex.
Why is the Kjeldahl method used?
To determine the amount of nitrogen from the organic and inorganic substances
Which catalyst is used during kjeldahl methods?
Generallycopper sulphate is used in these methods.
What are the three steps of these methods?
● Digestion
● Distillation
● Titration
Which compound is not suitable for kjeldahl method?
Those with N in Nitro and azo groups, as well as compounds with N in the ring (such as pyridine), are not amenable to Kjeldahl’s technique because N in these compounds does not transform to other forms (NH4)2SO4
Which gas is liberated during the Kjeldahl method?
Ammonia is liberated during the kjeldahl method.
Can Kjeldahl method use pyridine?
No , because these compounds don’t transform into other forms.






