Table of Contents
ToggleCrystalline solid and amorphous solid is the form of solids which are distinguished on the basis of the geometric patterns of their constituents, such as atoms, molecules, or ions. Solids, rigid substances, are characterized by definite shape, incompressibility, and mechanical strength. Unlike gases and liquids, the molecules, atoms, or ions that make up a solid are closely packed i.e. they are held together by strong forces and hence are unable to more randomly. The properties of solids are determined not only by the number and their constituents/compositions but also by their arrangements.
Solids are broadly categorized into two types:
- Crystalline solids
- Amorphous solids
Amorphous solid and Crystalline solid
Amorphous solids
An amorphous solid is a substance whose constituents, such as atoms, molecules, or ions are not arranged in a regular pattern over a long-range. They are not in the form of definite geometric patterns i.e. lacks the ordered crystalline lattice. Some examples of amorphous solids are rubber, plastics, starch, glass, and so on.
Crystalline solids
A crystalline solid is a substance whose constituents, such as atoms, molecules, or ions are arranged in a regular pattern, which repeats itself over long-range in a three-dimensional fashion. They are in the form of definite geometric patterns. Some examples of crystalline solids are sugar, sulphur, salt, diamond, quartz, and so on.
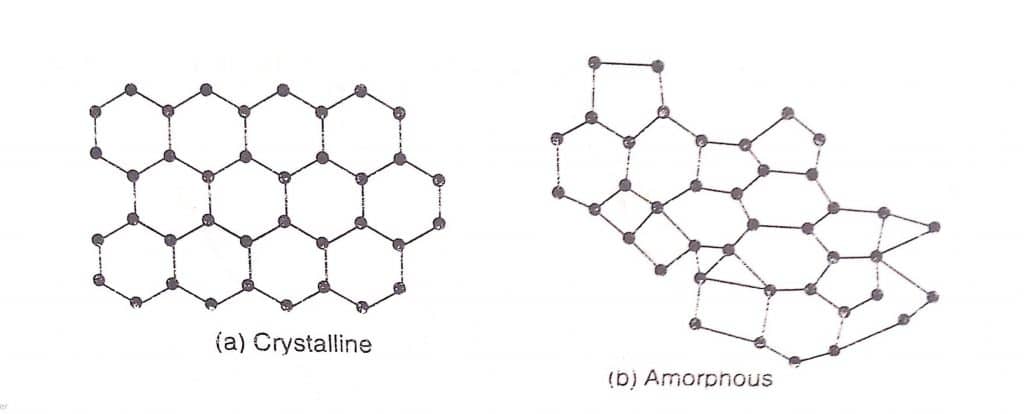
Difference between Crystalline and Amorphous solid
| Crystalline solids | Amorphous solids |
| A crystalline solid is a substance whose constituents, such as atoms, molecules, or ions are arranged in a regular pattern, which repeats itself over long-range in a three-dimensional fashion. | An amorphous solid is a substance whose constituents, such as atoms, molecules, or ions are not arranged in a regular pattern over a long-range. |
| Have a definite geometrical shape. | They do not have a definite geometrical shape. |
| Have a sharp melting point. | Do not have a sharp melting point. |
| Definite heat of fusion. | They do not have definite heat of fusion. |
| They are true solids. | They are regarded as pseudo solids or super cooled liquids. |
| Crystalline solids are anisotropic in nature as their physical properties are different in different directions. | Amorphous solids are isotropic since they have the same physical properties in all directions. |
| Sugar, sulphur, salt, diamond, quartz, etc. are some examples of it. | Examples of amorphous solids are rubber, plastics, starch, glass, and so on. |
Isotropy and Anisotropy
Crystalline solids are anisotropic since. their physical properties such as refractive index, chemical bonding, thermal and electrical conductivity, etc. are different in different directions. Thus, anisotropy is referred to as the properties of a substance that is direction oriented i.e. different properties in different directions. Some of the examples of anisotropic substances/solids are composite materials, woods, crystals (except cubic crystals), and so on.
Amorphous solids are called isotropic because their physical properties such as refractive index, conductivity, and mechanical strength are the same in all directions. Thus, isotropy is referred to as the properties of a substance which is independent of the direction i.e. same physical property in all directions. Some examples of isotropic substances/solids are diamonds, glass, metals, and so on.
Amorphous solid and Crystalline solid Video
FAQs/MCQs
Example of crystalline solid and amorphous solid
Sugar, sulphur, salt, diamond, quartz, etc. are some of the examples of crystalline solids, while examples of amorphous solids are rubber, plastics, starch, glass, and so on.
What is crystalline solid and amorphous solid?
A crystalline solid is a substance whose constituents, such as atoms, molecules, or ions are arranged in a regular pattern, which repeats itself over long-range in a three-dimensional fashion.
An amorphous solid is a substance whose constituents, such as atoms, molecules, or ions are not arranged in a regular pattern over a long-range.
References
- Arun Bahl, B. S. Bahl & G. D. Tuli, Essentials of Physical Chemistry, S. Chand and Company Ltd., New Delhi, 2012.
- A R West, Solid State Chemistry and its Applications.






