Table of Contents
ToggleDifference between ideal gas and real gas has been discussed in this post. Before differentiating between these, one must have some knowledge of ideal gas and real gas. Ok, let’s explain ideal gas, real gas, and their related terms.
Ideal gas and Real gas
An ideal gas is one that obeys the gas equation and other gas laws under all conditions of temperature and pressure. These are known as a perfect gas.
A gas that does not obey the gas equation and all other gas laws strictly but tends towards ideality at low pressure and high temperature is known as a real gas.
The concept of an ideal gas is only theoretical or hypothetical and none of the known gases obeys the gas laws or gas equation. The gas behaves in a nearly ideal manner at low pressure and high temperature.
All known gases are real gases, actually ideal gas does not exist. But many scientists studied the behavior of the real gases under the different conditions of temperature and pressure and found out that all gases showed deviation from ideal behavior. Moreover, they found that the deviation is more at high pressure and low temperature.
We have just discussed that real gases show deviation from the ideal behavior. Then, what is the cause of such deviation from the ideal behavior? We will discuss it in another post.
Compressibility factors
For an ideal gas, PV=nRT
But, the gas equation can be modified for the real gas as,
PV=ZnRT
Where Z is a correction factor known as the compressibility factor. Thus, the compressibility factors can be expressed as:
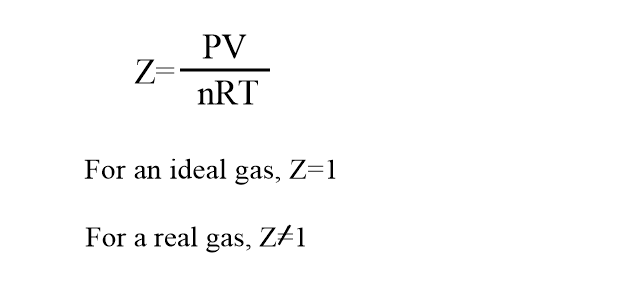
Note:
- The deviation of real gas from ideal gas is measured by the deviation of compressibility factors(Z) from unity.
- The deviation depends upon the temperature and pressure as well as the gas.
- The compressibility factor depends on temperature and pressure.
Boyle’s temperature
The point or temperature at which Boyle’s law remains valid over a certain range of pressure is known as Boyle’s temperature.
Difference between ideal gas and real gas
The major difference between the ideal gas and real gas is that the ideal gas follows the ideal gas equation under all conditions of temperature and pressure but real gas does not obey the ideal gas equation under all conditions of temperature and pressure. Some of the major differences between ideal gas and real gas are shown below:
| Ideal gas | Real gas |
| An ideal gas is one that obeys the gas equation and other gas laws under all conditions of temperature and pressure | A gas that does not obey the gas equation and all other gas laws strictly but tends towards ideality at low pressure and high temperature is known as a real gas. |
| The value of the compressibility factor is unity. | The value of the compressibility factor is not equal to unity. |
| Ideal gas does not exist. | All known gases are real gas. |
| The value of molar volume at NTP is equal to 22.4 L for ideal gas. | The value of the molar volume of real gas at NTP is not equal to 22.4 L. |
| Ideal gas follows the ideal gas equation and the ideal gas is supported by this equation. PV=nRT | A real gas is supported by Vander Waal’s equation. |
References
- Arun Bahl, B. S. Bahl & G. D. Tuli, Essentials of Physical Chemistry, S. Chand and Company Ltd., New Delhi, 2012.
- J. N. Gurtu and A. Gurtu, Advanced Physical Chemistry Experiments, (6th Edition), Pragati Prakashan, Meerut, India, 2014.