Table of Contents
ToggleThis blog offers a detailed discussion of colloids, their examples, properties, types, and importance. The word ‘colloids‘ is derived from greek words; Kolla = glue; eidos = like. A colloid is one of the three main categories of mixtures; the other two are a solution and a suspension.
Depending on the degree of dispersion, the systems are classified as 1) true solution, 2) colloidal solution, and 3) suspension when a dispersed phase is disseminated in a dispersion medium. The substance that is dispersed is said to be in the dispersed phase, whereas the substance that it is dispersed in is said to be in the continuous phase.
The size of the solute particle affects the type of solution that forms. If the solute particles are dispersed in the solvent as single molecules or ions and have a diameter ranging from 1Å to 10 Å., they will form a true solution. On the other hand, if the dispersed particles are aggregates of millions of molecules with a diameter of these particles in the order 1,000 Å or more, it forms a suspension.
A colloid is a solution that contains particles with a diameter between 1 to 100 nm, or 10 Å – 1000 Å, yet is still able to maintain their uniform distribution. Thus, the colloidal solution is referred to as an intermediate between true solutions and suspensions.
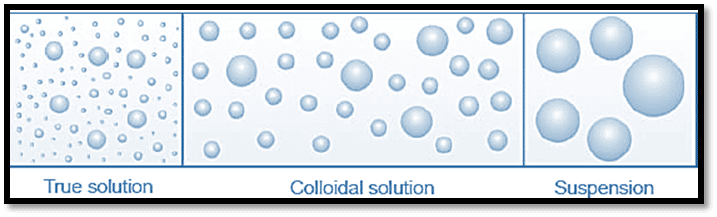
Properties of true solution, colloids, and suspension
| Property | True solution | Colloidal solution | Suspension |
| Size | 1Å to 10 Å (less than 1 nm) | 10 Å to 1000 Å (1 nm – 100 nm) | More than1000 Å (greater than 100 nm) |
| Appearance | Clear | Generally clear | Opaque or cloudy |
| Nature | Homogeneous | Heterogeneous | Heterogeneous |
| Visibility | Invisible to a microscope | Visible using an ultramicroscope | Visible to the naked eyes |
| Settling | Do not settle on itself | Can be settled by centrifugation | Under the influence of gravity, particles naturally settle. |
| Filterability | Pass through both animal membrane and ordinary filter paper | Filtration by ordinary filter paper, but not by animal membrane | Do not pass through an animal membrane or filter paper. |
| Diffusion | Diffuse quickly | Diffuse slowly | Do not diffuse |
| Separation | Not separated by ordinary filtration or by ultra-filtration | Not separated by ordinary filtration, but can be separated by ultra-filtration. | Can be separated by ordinary filtration. |
| Brownian motion | Do not occur | Occurs | May occur |
What are Colloids?
Any substances that are intermediate between true solutions and suspensions, and have the diameter of the particles of a substance dispersed in a solvent ranging from about 10 Å – 1000 Å, are called colloids. The material with particle size in the colloidal range is said to be in the colloidal state. The colloidal particles could be rod-like, disc-like, thin films, or lengthy filaments, rather than just corpuscular in shape.
Examples of colloids
Milk, cream, fog, butter, ice cream, froth, aerosols, paints, synthetic polymers, shoe polish, muddy water, smoke, dust in the air, cheese, soap bubbles, blood, gold sol, cloud, etc. are some of the examples of colloids.
Properties of Colloids
- Tyndall effect: The Tyndall Effect, a phenomenon in which light beams incident on colloids are scattered as a result of the interactions between the light and the colloidal particles, is known to occur in colloidal solutions.
- Brownian movement: Colloidal particles shows zing-zag movement either in a continuous or random manner. This motion is called the Brownian movement or Brownian motion and was first investigated by Robert Brown. Brownian motion arises because of the impact of the molecules of the dispersion medium on the particles of the dispersed phase. The forces are unequal in different directions. Hence it causes the particles to move in a zig-zag way
- Electrical properties: The dispersion medium has an equal and opposite charge to that of the colloidal solution’s particles, which all carry the same kind of charge. The solution as a whole is electrically neutral because the charge on the dispersion medium balances the charge on the dispersed particles. As a result, the colloidal particles repel one another and do not settle in a cluster. Based on the charge, colloidal sols may be classified as positively and negatively charged sols.
- Heterogenous in nature
Types of Colloids
Colloids are heterogeneous in nature consisting of two-phase; the dispersed phase and the dispersion medium. Dispersed phase is such a particle that is present in small proportions and has a colloidal size (1 to 100 nm) diameter. Dispersion medium, on the other hand, is such a medium in which colloidal particles are dispersed. These two phases may be either solid, liquid, or gas.
Depending on the dispersed phase and dispersion medium, different types of colloids are:
| Dispersed phase | Dispersion medium | Types of colloids | Examples |
| Solid | Solid | Solid sol | Gemstones, Ruby glass, Alloys |
| Solid | Liquid | Sol | Paints, cell fluids, gold sol, muddy water, starch sol |
| Solid | Gas | Aerosol of solids | Smoke |
| Liquid | Solid | Gel/Solid emulsion | Jellies, Cheese, Pearl |
| Liquid | Liquid | Emulsion | Milk, Cream, oil in water |
| Liquid | Gas | Aerosol | Mist, cloud, fog, |
| Gas | Solid | Solid foam | Foam rubber, Lava, pumice stone |
| Gas | Liquid | Foam | Froth, Soap suds, whipped cream |
Among the various types of colloids mentioned above; sols, gels, and emulsions are among the common colloids.
Classification of colloids
Classification based on molecular size
- Macromolecular colloids: The size of the dispersed phase particles in these colloids lies between 1–100 nm. Starch, cellulose, proteins, etc., are a few examples of naturally occurring macromolecular colloids.
- Multi-molecular colloids: Although the atoms are not colloidal in size, they combine together to form a molecule with colloidal dimensions. Example: Sulfur sol includes clusters of S8 molecules that are colloidal in dimension.
- Associated colloids: These substances exhibit normal electrolyte behavior at low concentrations, but as concentrations are raised, they form micelles and exhibit colloidal solution behavior. Soap is one example of associated colloids.
Classification based on interaction
- Lyophilic colloids: When the dispersed phase of a colloidal solution exhibits a strong affinity (or love) for the dispersion medium, the solution is referred to as lyophilic.
- Lyophobic Colloids: When a dispersed phase has no affinity for the dispersion medium, a colloidal solution is said to be lyophobic.
Differences between lyophilic and lyophobic colloids
| Lyophilic colloids | Lyophobic colloids |
| The dispersed phase has a high affinity for the dispersion medium | The dispersed phase has very low or no affinity for the dispersion medium. |
| Solvent-loving colloids. | Solvent-hating colloids. |
| Reversible in nature. | Irreversible in nature. |
| They are stable colloids. | Can be stabilized by a stabilizer. |
| Form lyophilic sol | Form lyophobic sol |
| Do not exhibit Tyndall effect | Exhibit Tyndall effect |
| Have surface tension lower than that of the medium. | Surface tension is the same as that of the medium. |
| Viscosity is higher than dispersion medium. | Viscosity is almost the same as that of the medium. |
| Precipitated by high electrolyte concentration. | Precipitated by low electrolyte concentration. |
| No or little charge on particles | Particles acquire positive or negative charges. |
Application of colloids
Some of the major applications of colloids are:
- Water Purification
- Clotting of Blood
- Blue Colour of the Sky
- Smoke Precipitator
- Artificial Rainfall
- Pumping of slurries, coating technology, caking, powder flow, and filtration via the sintering principle
- Photographic products
- Radioactive waste disposal
- Wettings of powders
- Pollution control
- Catalysts
- Pharmaceuticals, cosmetics, inks, paints, foods, lubrication, food products, dyestuffs, foams, agriculture chemicals, and so on.






