Table of Contents
ToggleThe determination of molecular weight of polymers can be carried out using three methods; Viscosity method, Osmotic pressure method, and Light scattering method.
Polymers are long-chain high molecular weight compounds obtained by the combination of a large number of simple molecules called monomers. This process of joining together many small molecules (monomers) to form a very large molecule (polymer) is called polymerization.
The molecular weight is an important property of polymeric substances. Generally, molecules of a protein or a polymer may not be of the same size, and hence the molecular weight determination will give some kind of average value.
Determination of molecular weight of polymers by Viscosity Method
The viscosity of the solution increases when a solute is added to a pure solvent. In a similar way, adding polymer to a solvent makes it more viscous than a solvent that is pure. The relative viscosity of a solution of polymer, ηr is given by
ηr = η /ηo ………………………(i)
Where, η =viscosity of the solution, and ηo = viscosity of the pure solvent
The specific viscosity, ηSP is given by
ηSP = ηr – 1 …………………(ii)
Considering equations (i) and (ii), the intrinsic velocity can be defined as
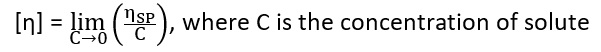
A straight line is obtained on plotting ηSP/C Vs C. The extrapolation of the line (C→0) gives the intercept equal to intrinsic velocity.
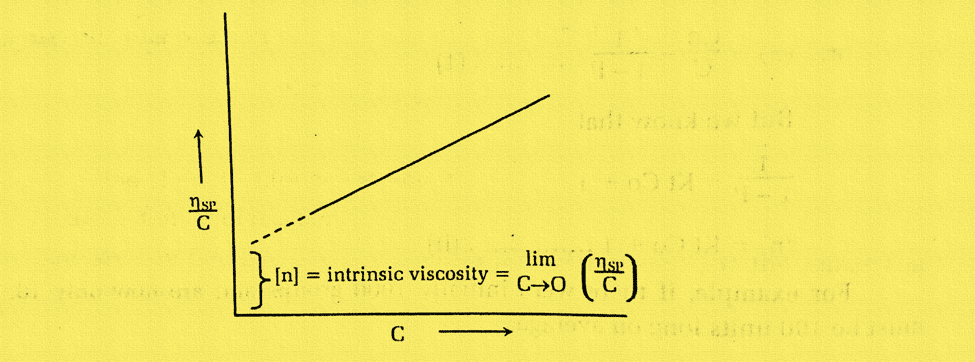
Staudinger demonstrated that there is an empirical correlation between the intrinsic viscosity of a polymer and its molecular weight.
[η] = KMa ………………..(iii)
Where “K” and “a” are constant for a specific polymer in a specific solvent. If these constants are known, then the Molecular weight of polymers (M) can be calculated from the determination of the value of intrinsic velocity.
Determination of molecular weight of polymers by Osmotic Pressure Method
Osmotic pressure is another well-known method for the determination of the molecular weight of macromolecules/polymers.
The Van’t Hoff equation for a dilute solution is
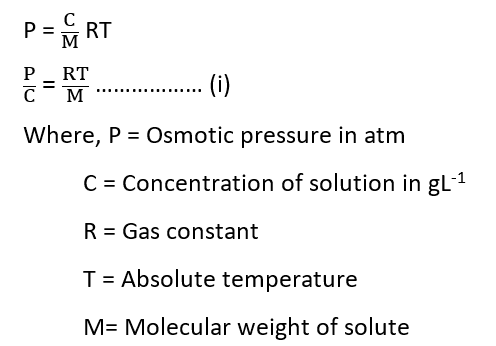
Here, an osmometer is used to measure the osmotic pressure (P) of a number of small concentrations (C). On plotting P/C vs. C, a straight line is obtained. We get an intercept RT/M, from which the molecular weight can be calculated when this plot is extrapolated to zero concentration (C→0).
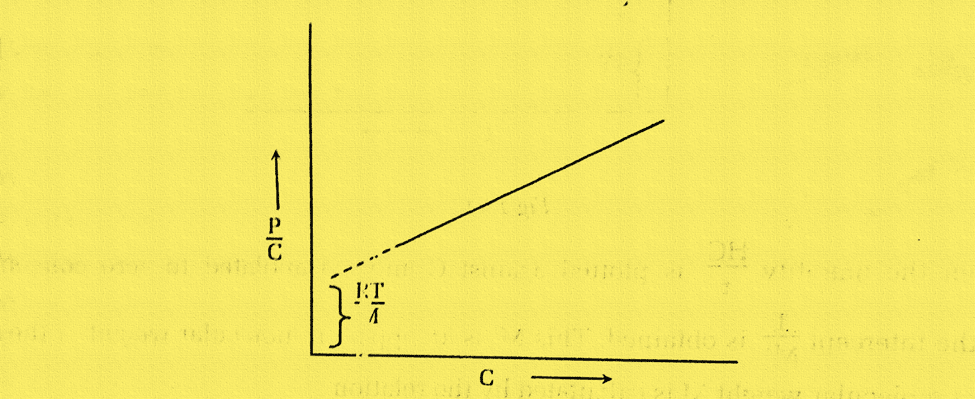
Determination of molecular weight of polymers by Light Scattering Method
Light scattering originates when polarizable particles are positioned in a beam of light’s oscillating electric field. When light passes through a polymer solution, energy is lost due to absorption, transformation to heat, and dispersion or scattering, which is measured by the light scattering method.
The following relationship governs how to determine the molecular weight of polymers in solution by measuring the intensity of scattered light:
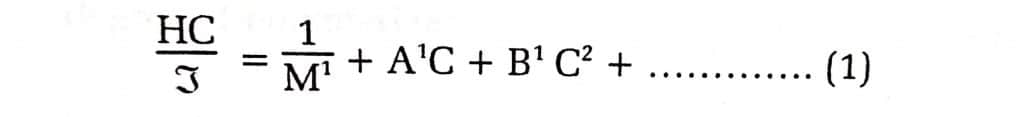
Where, C= concentration of the solution in gm cm-3, and A1 and B1 are constant quantities.
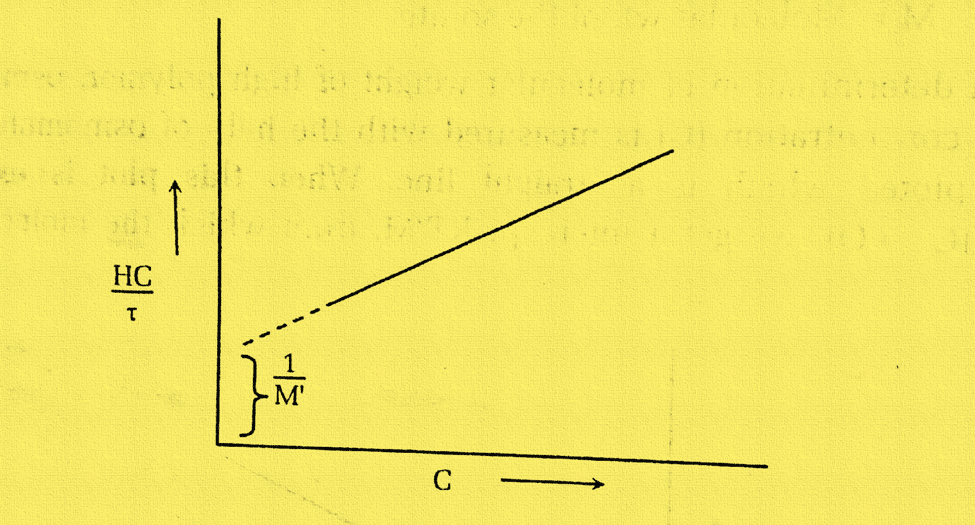
The intercept 1/M1 is obtained when the quantity HC/τ is plotted against C and extrapolated to zero concentration. The apparent molecular weight of the solute is represented by this M1. The correct molecular weight M is calculated by the relation
M = M1 α β
The quantities α (dissymmetry correction) and β (depolarization correction) are correction terms necessitated by the size and nature of the solute molecules. In this way, the molecular weight of polymers can be determined.