Table of Contents
ToggleGels are jelly-like colloidal systems in which a liquid is dispersed in a solid medium. The majority of commonly used gels are hydrophilic colloidal solutions, in which a diluted solution is formed as elastic semi-solid masses under appropriate conditions. Despite the fact that the liquid makes up the majority of their (gels) mass, they have solid-like characteristics including non-zero yield stress.
For instance, when a warm gelatin sol is cooled, the gel-like semisolid mass forms. This process of gel formation is called gelation. Gelation can be compared to a sol that has partially coagulated. Firstly, the coagulating sol particles combine to create lengthy chains that resemble threads. Then, these chains are linked together to create a solid structure. The cavities of this structure trap the liquid dispersion medium resulting in the semisolid porous material called gel.
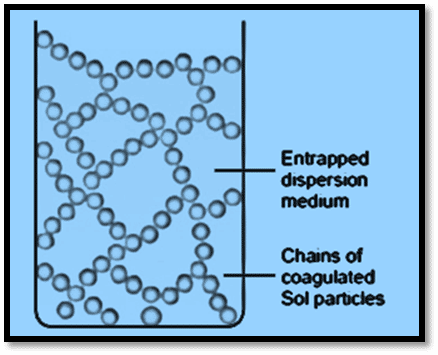
Sols and gels are quite interrelated terms as they both are lyophilic colloids. Gel is a solid or semisolid state of colloidal solution, while sol is its liquid state. Both protein and starch can be used in the form of sol or gel.
Gels may shrink on long-standing by losing some of the liquid they were holding. This process is known as Syneresis. Gels have been found useful from a cosmetics perspective to building materials. It is also used in the pharmaceutical industry along with various fields.
Examples of Gels
Boot polish, cheese, jelly, curd, silica, etc. are some common examples of gel.
Types of Gels
Gels may be categorized into two types:
- Elastic gels: Gels that exhibit elasticity are known as elastic gels. When a force is applied, they change shape, but when the force is released, they return to their previous shape. In general words, they return to their previous state when water is added after becoming somewhat dehydrated from losing water. Cooling relatively concentrated lyophilic sols result in elastic gels. Elastic gels can be formed by a variety of substances, including starch, gelatin, and soaps. By cooling relatively concentrated lyophilic sols, elastic gels can be produced. The electrical attraction that links the molecules together results in flexible connections.
- Non-elastic gels: Gels that are rigid, like silica gel, are considered non-elastic gels. These are prepared using the proper chemical process. Thus, sodium silicate solution of the proper concentration is treated by adding strong hydrochloric acid to generate silica gel. The resultant silicic acid molecules polymerize to create silica gel. It has a network of covalent bonds connecting it, giving it a strong, rigid structure. The non-elastic gels are not reversible like elastic gels.
Properties of gels
Gels are semi-solid materials with characteristics ranging from soft and brittle to strong and tough.
- Hydration: Water can be added to renew an elastic gel that has fully lost all of its moisture. However, once a nonelastic gel is dry, adding more water won’t cause it to gel.
- Swelling: Elastic gels that have been partially dehydrated absorb water when immersed in the solvent. This results in an increase in the gel’s volume, a process known as swelling.
- Syneresis: On standing, many inorganic gels shrink, and this shrinkage is often accompanied by solvent exudation. This process is called syneresis.
- Thixotropy: When at rest, some gels are semisolid; yet, when disturbed, they become liquid sol. This reversible sol-gel transformation is referred to as Thixotropy. Gels made of iron oxide and silver oxide exhibit this characteristic.
Gels Video
References
- Arun Bahl, B. S. Bahl & G. D. Tuli, Essentials of Physical Chemistry, S. Chand and Company Ltd., New Delhi, 2012.
- Paul C. Hiemenz and Raj Rajagopalan, Principles of colloid and surface chemistry.
- Introduction to Applied Colloid and Surface Chemistry; Georgios M. Kontogeorgis, Søren Kiil.
- Khademhosseini A, Demirci U (2016). Gels Handbook: Fundamentals, Properties, and Applications.
- K. S. Birdi – Handbook of Surface and Colloid Chemistry






