Table of Contents
ToggleSurface tension, one of the important properties shown by liquids, is due to the intermolecular forces of attraction. It depends not only on the attraction of forces between the particles within a liquid but also on the force of attraction of a solid, liquid, or gas that is in contact with it. Thus, it is a phenomenon that occurs when the liquid’s surface comes into contact with another phase (solid, liquid, or gas).
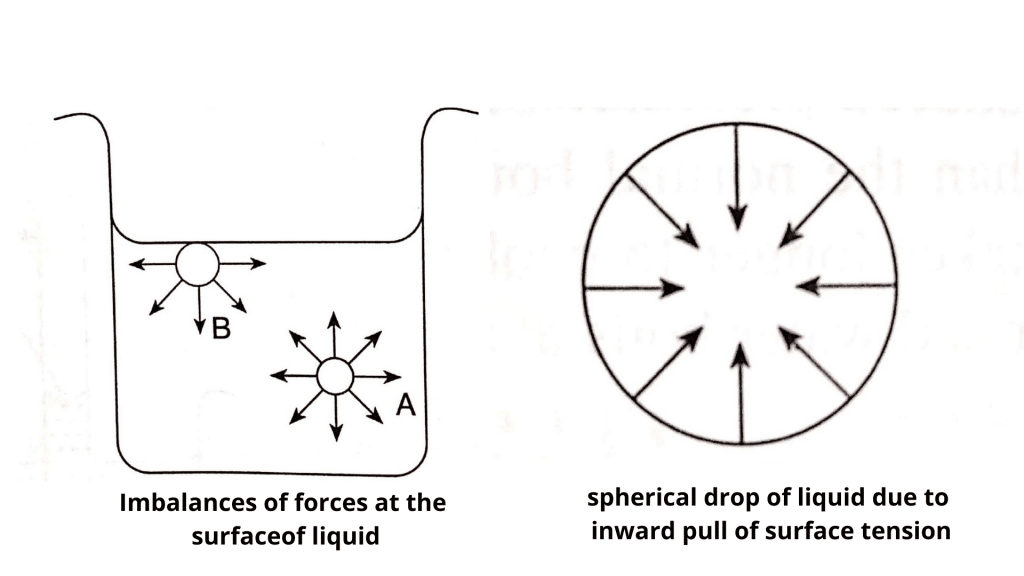
Let us consider a molecule ‘A’ in the interior of liquid that is equally attracted in all directions by the surrounding molecules, and hence is in the state of balanced attractions. On the other hand, a molecule like ‘B’ on the liquid’s surface, is only attracted by molecules below and beside it. Since downward attractive forces are greater than upward forces, the molecules at the surface are pulled inwards and the surface area of the liquid tends to be minimum. This is the reason drops of liquid in the air take spherical shapes as a sphere possesses the smallest surface area for a given volume.
Surface tension is a property of a liquid’s surface layer that causes it to behave as an elastic sheet.
Surface tension definition
Surface tension (γ) is defined as, “The tension in a liquid’s surface film caused by the attraction of particles in the surface layer by the bulk of the liquid, which tends to minimize surface area“. In general words, surface tension is also defined as the force that acts at right angles to an imaginary line of unit length at the surface of the liquid at rest or the force acting in dynes along the surface of a liquid at right angles to any line 1 cm in length.
Mathematically, surface tension is
Surface tension (γ) = F/L, where F is a force acting in dyne and L is the length.
Units of surface tension
The unit of surface tension (γ) in CGS system is dynes per centimeter (dyne cm–1), while is Newton per meter (Nm–1) in the SI system.
Examples of surface tension
Some of the examples of surface tension are:
- Spherical shape of small drops of liquids and soap bubbles.
- Insects walking on the surface of the water.
- Cleansing action of soaps and synthetic detergents.
- Floating of a needle on the surface of water.
- Filling a water glass above the rim.
- Filling ink in the nib of a pen
Effects of temperature on surface tension
The surface tension of a liquid decreases with an increase in temperature. This is because when the temperature increases, the kinetic energy of liquid molecules also increases since K.E. ∝ T, which in turn decreases the intermolecular attractions. Hence, surface tension decreases with an increase in temperature.
Consequences of surface tension
- Spherical shape of liquid drops: The spherical shape of liquid drops is due to surface tension as it tends to minimize the surface area; the sphere possesses the smallest surface area for a given volume.
- Capillary action: The surface tension of the liquid is responsible for capillary action. When the adhesive forces (the attractive force between molecules of different substances) are greater than the cohesive force (the attractive force between molecules of the same substances), the molecules of the liquid are attracted more strongly by the molecules of the gas capillary because of which liquid rises in the capillary forming concave meniscus. E.g. water or oil. But, when cohesive forces are greater than adhesive forces, the convex meniscus is formed as the level of liquid inside the capillary is lower than the level outside. E.g. mercury.
- Cleansing action of soap and detergents: Soaps and detergents lower interfacial tension between water and grease which leads to the emulsification of grease in water, and hence the dirt that sticks to the greases are washed away.
- Efficiency of toothpaste: This is due to the lowering of surface tension that leads to spreading over the surface they come in contact with.
Surface tension and Viscosity
Surface tension is the tension in a liquid’s surface film caused by the attraction of particles in the surface layer by the bulk of the liquid, which tends to minimize surface area It is expressed in dyne cm–1 in CGS units or Nm–1 in the SI system. The surface tension of liquid decreases with an increase in temperature due to a decrease in intermolecular attraction. It can be determined using capillary-rise method, drop formation method, Ring-detachment method, and so on. Some of the examples include the spherical shape of a liquid drop, cleansing acting of soaps and detergents, capillary action, etc,
Viscosity is the force of attraction between two layers of liquid moving past one another with different velocities. It is expressed in dynes sec/cm-2 (CGS units) or N sec/m-2 (SI unit). Viscosity of liquid decreases with an increase in temperature but the viscosity of gas increases with an increase in temperature. The reciprocal of the viscosity is called fluidity. It can be determined using Ostwald method or viscometers. Some examples of viscosity include cooking oil, honey, paints, lubricants, etc.
Surface tension and Surface energy
Surface tension measures the force per unit length of the surface and depends not only on the attraction of forces between the particles within a liquid but also on the force of attraction of a solid, liquid, or gas that is in contact with it. The term surface tension refers to the attractive forces of liquid molecules.
Surface energy measures the amount of work that needs to be done per unit area in order to stretch it. Due to the intermolecular forces between molecules, work needs to be done on a surface in order to stretch it. Surface energy is commonly used to describe the attractive force between molecules of a solid substance.
Surface tension video
FAQs/MCQs
Effect of impurities on surface tension
Impurity that is extremely soluble like salt in water increases the surface tension of the water, while Insoluble impurities, such as detergent, reduce the surface tension of water.
the surface tension of water
The surface tension of water is 0.07275 joule per square meter at 20 °C (68 °F).
what causes surface tension
Cohesive interactions between the molecules in the liquid cause surface tension.
surface tension formula
surface tension formula is T = F/L, where T is surface tension, F is force, and L is length.
water has surface tension because …
water has surface tension because hydrogen bonds between surface water molecules resist being stretched.
experiment that deals with surface tension
experiment that deals with surface tension involve capillary-rise method, drop formation method, Ring-detachment method, and so on
Is surface tension adhesion or cohesion
Surface tension arises due to cohesive interactions between the molecules in the given liquid.
surface tension and intermolecular forces
Surface tension, one of the important properties shown by liquids, is due to the intermolecular forces of attraction. Surface tension is higher in liquids with stronger intermolecular forces than in those with weaker intermolecular forces.
surface tension and fluidity
Surface tension is the force acting in dynes along the surface of a liquid at right angles to any line 1 cm in length. The reciprocal of viscosity is called fluidity.
surface tension of ethyl acetate
surface tension of ethyl acetate is 0.024 N/m at 25oC (77oF).
surface tension of water with surfactant
By adsorbing at the liquid-gas interface, the surfactant lowers the surface tension of water.
which best explains the surface tension of water
Cohesion between the molecules explains the surface tension of water.
surface tension of ethanol
surface tension of ethanol is 22.32 dyne/cm at 20°C.
why does detergent decrease the surface tension of water
One end of a detergent is polar, whereas the other is hydrophobic (water-hating). The detergent’s polar end can form bonds with polar water molecules, lowering the water’s surface tension. This is advantageous when washing clothes since dirt and grease may be removed using water and detergent. Water alone does not clean well because the water molecules are too attracted to each other.
how does polarity affect surface tension?
The polar characteristic of water causes water molecules to attract one another. Water “sticks” together because the hydrogen ends are positive compared to the negative oxygen ends. This is why there is surface tension because breaking these intermolecular interactions requires a certain amount of energy.
does water temperature affect surface tension?
Surface tension decreases with an increase in surface tension.
n-butanol surface tension
n-butanol surface tension is 0.025 N/m at 25oC (77oF).
octane surface tension
octane surface tension is 0.021 N/m at 25oC (77oF).
surface tension of mercury
surface tension of mercury is 0.485 N/m at 25oC (77oF).
decane surface tension
decane surface tension is 0.024 N/m at 25oC (77oF).
methanol surface tension
methanol surface tension is 0.022 N/m at 25oC (77oF).
surface tension symbol
surface tension symbol is γ.
glycerol surface tension
glycerol surface tension is 0.064 N/m at 25oC (77oF).
hexane surface tension
hexane surface tension is 17.91 dyn/cm at 25°C.
surface tension of acetone
surface tension of acetone is 23.32 dyne/cm at 20°C.
surface tension of chlorobenzene
surface tension of chlorobenzene is 33.28 dyne/cm at 20°C.
does water have a high surface tension
Water has a higher surface tension than most other liquids due to the relatively high attraction of water molecules to each other through a web of hydrogen bonds.
dimension of surface tension
dimension of surface tension is [MT−2].
does surface tension increase with temperature
No, surface tension decrease with an increase in temperature.
how does sugar affect the surface tension of water
The concentration of solutes in water increases when sugar is added to it. Chemical interactions between sugar and water molecules provide hydration energy. The H-bonding energy decreases as hydration energy increases (reduces the surface tension). Hence, Sugar reduces surface tension.
surface tension is a direct result of
surface tension is a direct result of cohesive interactions between the molecules in the liquid.
surface tension determination methods
surface tension determination methods are capillary-rise method, drop formation method, and so on.
surface tension of water is
surface tension of water is 0.07275 joule per square metre at 20 °C (68 °F).
why does surface tension decrease with increasing temperature
This is because when the temperature increases, the kinetic energy of liquid molecules also increases since K.E. ∝ T, which in turn decreases the intermolecular attractions.
References
- Halliday, David; Resnick, Robert; Krane, Kenneth S. (2010-04-20). Physics, Volume 2. John Wiley & Sons. p. 342.
- Bush, John W. M. (May 2004). “MIT Lecture Notes on Surface Tension, lecture 3” (PDF). Massachusetts Institute of Technology. Retrieved April 1, 2007.
- Arun Bahl, B. S. Bahl & G. D. Tuli, Essentials of Physical Chemistry, S. Chand and Company Ltd., New Delhi, 2012.






