Table of Contents
ToggleThe molecular velocities like average velocity, root mean square velocity, and most probable velocity are well discussed here:
Average velocity
Average velocity is the arithmetic mean of the various velocities of the molecules. It is symbolically represented by vav or v.
If c1, c2, c3, …………cn are the velocities of individual molecules in a gas and ‘n’ is the total number of molecules present in the gas, then the average molecular velocity is given by
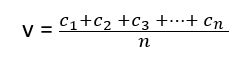
The value of average velocity calculated from Maxwell’s law of distribution is
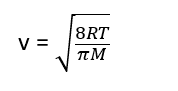
where R = Gas constant, T = Temperature (in Kelvin scale), and M = Molar mass of the gas
Thus by substituting the values of R, T, π, and M in the above equation, average velocity can be calculated.
Root mean square velocity
The square root of the mean of squares of each gas molecule’s velocity is known as the root mean square velocity (r.m.s). It is denoted by μ or vrms.
If c1, c2, c3, …………cn are the velocities of individual molecules in a gas, and ‘n’ is the total number of molecules present in the gas, then root mean square velocity (r.m.s.) is given by
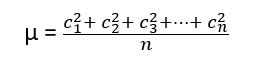
The Kinetic Gas Equation can be used to calculate the RMS velocity u at a given temperature as
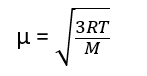
where R = Gas constant, T = Temperature (in Kelvin scale), and M = Molar mass of the gas
Thus, root mean square velocity can be calculated by putting the value of R, T, and M in the above equation.
Most Probable Velocity
Most probable velocity is defined as the velocity possessed by the largest number of molecules in a gas. It is denoted by vmp or α.
According to Maxwell’s law of distribution of molecular velocities, the most probable velocity is given by the expression.
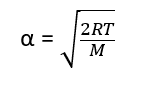
This is the required formula for most probable velocity of gas molecules.
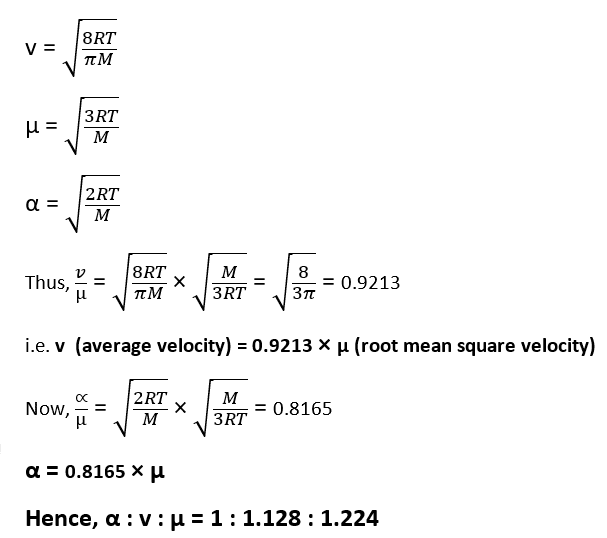
Relation between Average Velocity, Root mean square Velocity and Most Probable Velocity
As we know,
Molecular Velocity Video
FAQ/MCQs
What is average velocity of gas molecules?
Average velocity is the arithmetic mean of the various velocities of the molecules.
mean velocity of gas molecules
mean velocity of gas molecules is also called average velocity and is defined as the arithmetic mean of the various velocities of the molecules.
average velocity of gas molecules is proportional to
average velocity of gas molecules is proportional to the temperature.
What is molecular velocity?
The distance traveled by a molecule in a given gas per unit of time is known as molecular velocity.
Velocity distribution of gas molecules
Three types of velocities are considered in the study of the kinetic molecular theory of gases. They are average velocity, r.m.s, and most probable velocity.
References
- Raymond A. Serway; Jerry S. Faughn & Chris Vuille (2011). College Physics, Volume 1
- Arun Bahl, B. S. Bahl & G. D. Tuli, Essentials of Physical Chemistry, S. Chand and Company Ltd., New Delhi, 2012.






