Table of Contents
ToggleHeck reaction is a powerful tool for constructing a carbon-carbon bond in a single transformation using different types of aryl or vinyl halides and alkene. This reaction also tolerates a wide variety of functional groups, including alcohols, ethers, aldehydes, ketones, and esters. This feature contributes to the enormous synthetic utility of the Heck reaction. Richard heck discovered and developed this reaction in the late 1960s.
Heck reaction
It is a palladium-catalyzed coupling reaction between an aryl or vinyl halide and an alkene without allylic hydrogen. The typical catalyst is Pd(0)-phosphine complexes such as Pd(PPh3)4 or in situ catalysts such as Pd(OAc)2/PPh3. This reaction uses a palladium catalyst along with a phosphine ligand in presence of a base such as triethylamine. The base scavenges the produced hydrogen halide in the Beta-elimination step of the catalytic cycle. Therefore, a base is required in this reaction to remove liberated hydrogen halide.
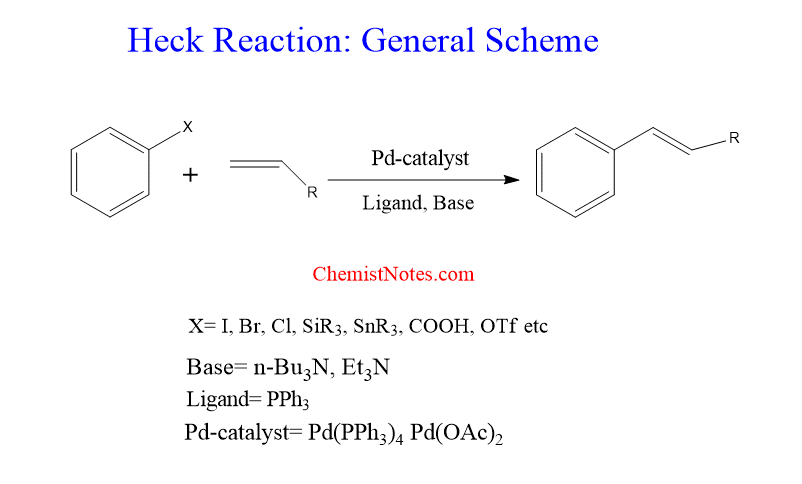
The organic halide should not have beta-hydrogens otherwise it will form olefins at the Pd(II) center.
Steric factors play important role in the regioselectivity of the addition of aryl-palladium complex to a substituted alkene. Electron withdrawing substituent attached at carbon-carbon double bond improves the regioselectivity while electron donor substituted alkene gives rise to the formation of regioisomers.
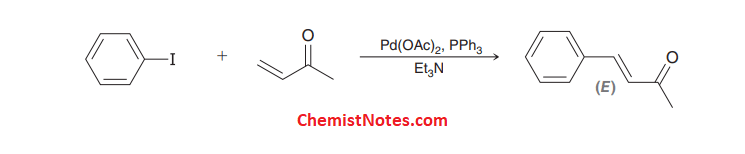
Heck reaction examples
Some examples of these reactions are given below:
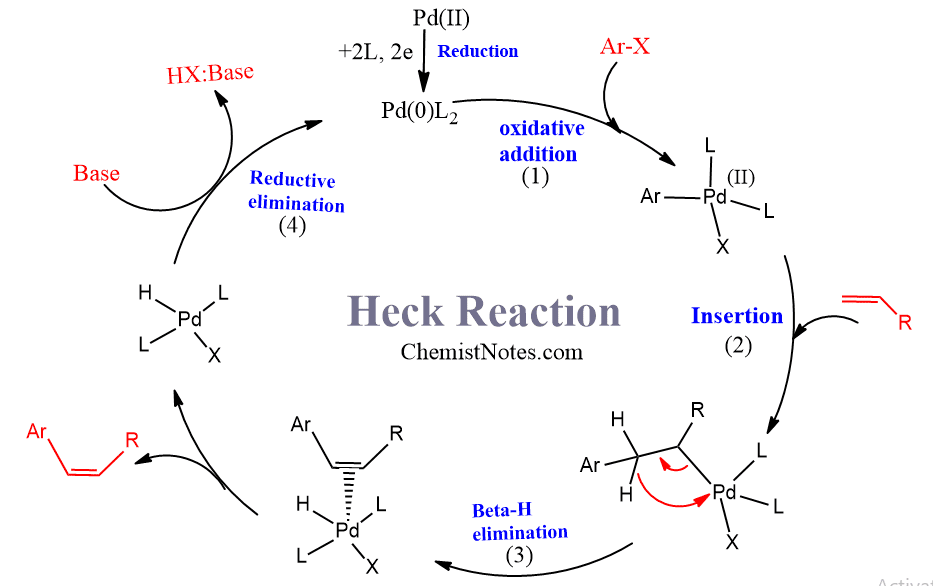
Mechanism of heck reaction
The active form of the catalyst is a `14 electron Pd(0) complex with two PPh3 ligands. The catalytic cycle of this reaction completes in the following four steps:
- Formation of four-coordinated aryl palladium(II) complex or intermediate, also known as palladacycle, by oxidative addition of the aryl derivative to Pd(0) complex.
- The addition of complex to the alkene. This is known as the alkene insertion reaction.
- Beta-hydrogen elimination
- Regeneration of Pd(0) complex by the reductive elimination.
The mechanism of this reaction is catalyzed by a neutral Palladium complex is shown below.
Intramolecular heck reaction
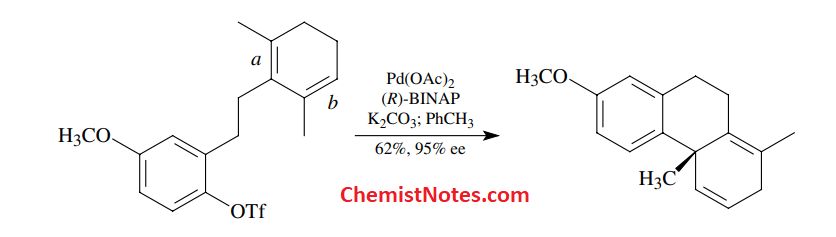
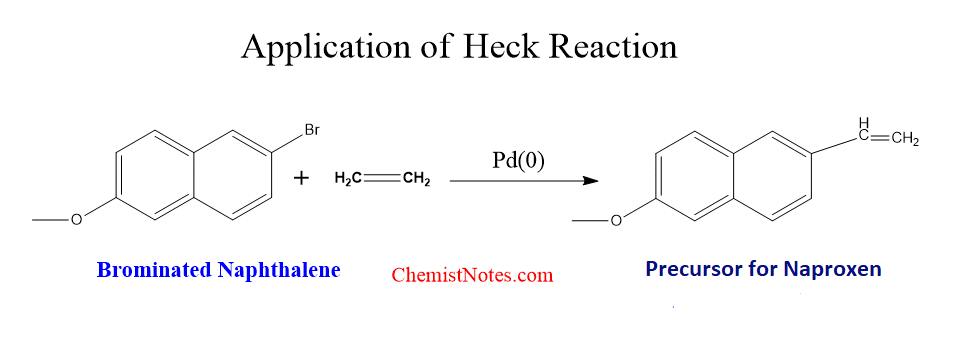
Application of Heck reaction
This reaction is very useful in the synthesis of natural products and biologically active compounds. More than 100 different such compounds have been synthesized using this reaction.
Naproxen is an anti-inflammatory drug, which has been synthesized on an industrial scale by using this reaction.
Moreover, other complex organic compounds such as Taxol, diterpenoids, etc can be synthesized.






