Table of Contents
ToggleGrubb’s catalyst and Schrock’s catalyst are very popular transition metal-carbene catalysts used in metathesis reactions. The general introduction, structure, advantages and disadvantages, and mechanism along with examples have been discussed in this post.
Grubb’s catalyst
Grubb’s catalyst is a ruthenium-carbene complex that was devolved by scientist Robert H. Grubbs in 1996. He had synthesized several generations of catalysts, a few of them are mentioned below.
Grubb’s first generation catalyst
The first catalyst introduced by him is known as Grubb’s first generation catalyst. The structure of a first-generation catalyst is shown below:
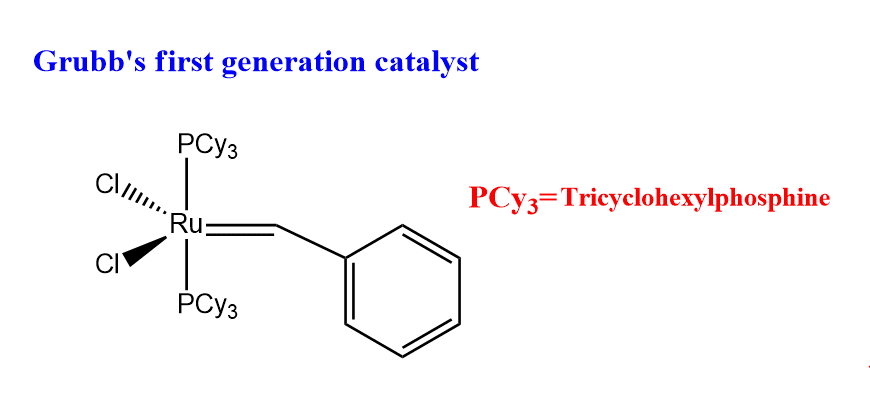
Advantages of Grubb’s first generation catalyst
- It shows low activity compared to schrock’s catalyst.
- It is easy to handle because it is stable to air and moisture.
- It shows good functional group tolerance to aldehyde, alcohol, and acids.
- Even a very low quantity of catalyst works well.
Disadvantages of Grubb’s first generation catalyst
- This catalyst cannot be recycled after use.
- Catalysts contamination of products observed.
- Ring-opening polymerization reaction only takes place in the case of strain rings.
- This catalyst is not suitable for the preparation of tri, tetrasubstituted olefins.
- This is not useful for deactivated olefins.
- Catalyst does not work well in presence of primary amines.
Let’s have an example of a reaction catalyzed by Grubb’s catalyst.
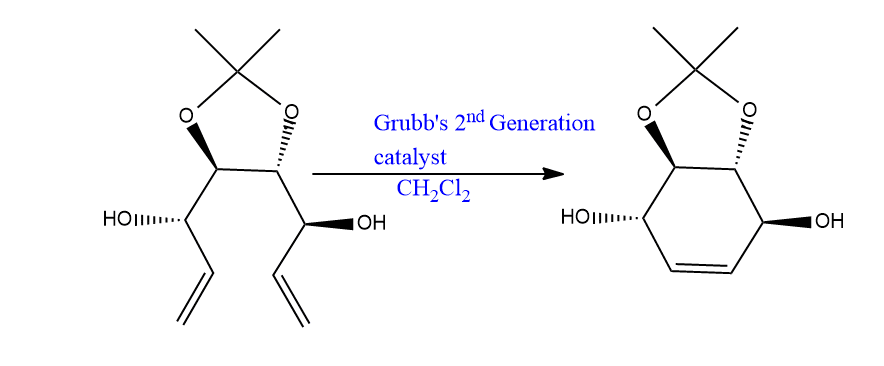
Grubb’s second generation catalyst
Ok, now let’s discuss Grubb’s second-generation catalyst which was introduced in 1999. One of the Pcy3 groups in Grubb’s first generation catalyst is replaced with an N-heterocyclic carbene ligand. The structure of the second-generation catalyst is shown below.
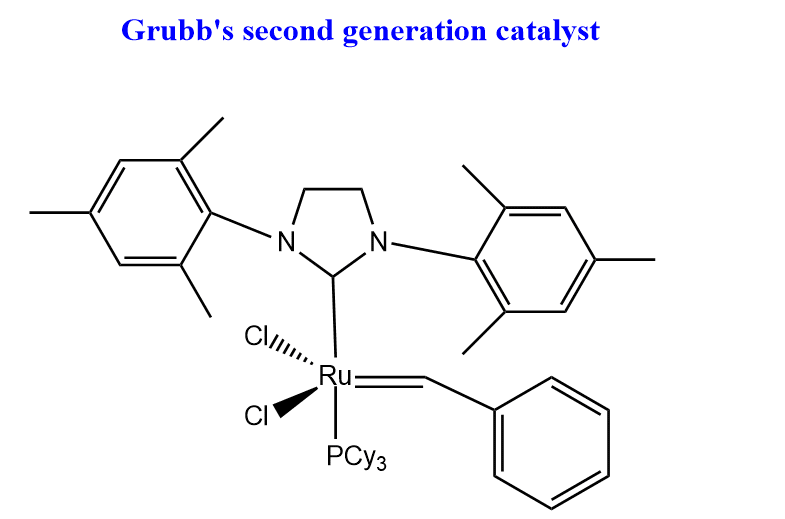
The second-generation catalyst of Grubb has higher activity than the first-generation catalyst. This catalyst shows good functional group tolerance to aldehyde, alcohol, and acids and it is easy to handle in air and moisture. Moreover, ring-closing metathesis of sterically bulky diene and deactivated olefins is possible by using this catalyst.
The limitations are the same as first-generation catalysts such as slow initiating, no recyclability, and poor tolerances to amines, thioethers, and phosphines.
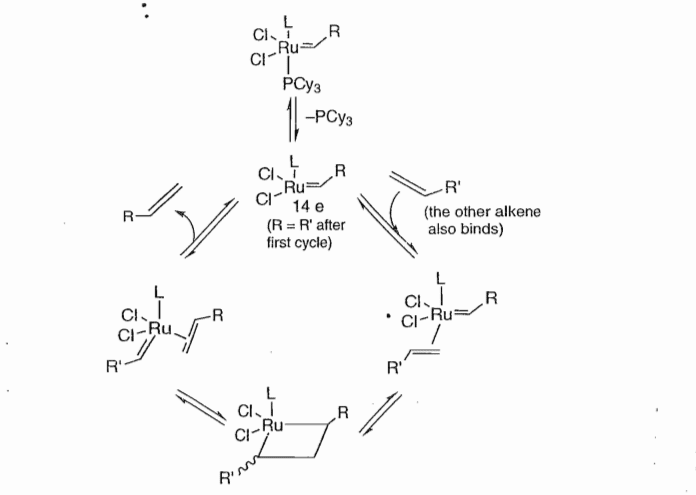
These catalysts catalyze the reaction by the following mechanism.
Hoveyda-Grubb’s catalyst II
The structure of Hoveyda-Grubb’s catalyst is shown below;
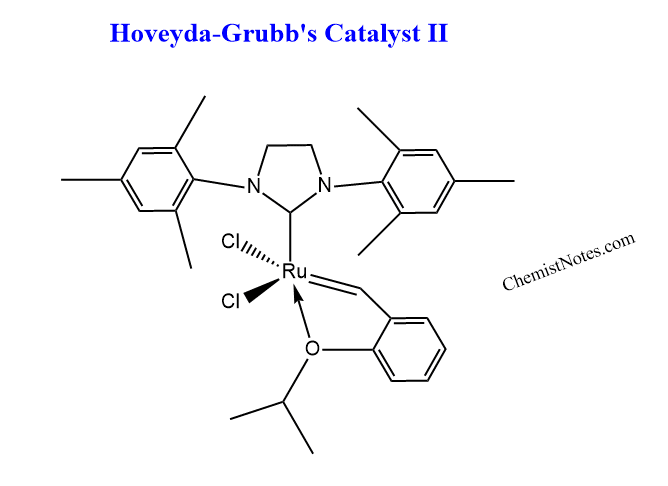
Advantages of Hoveyda-Grubb’s catalyst
- it is relatively easy to handle.
- it shows good functional group tolerance.
- it is stable in air and moisture.
- Catalyst can be recovered and reused
- it has improved activity than Grubb’s 2nd generation to electron-deficient olefins.
- The catalyst is useful for the synthesis of trisubstituted olefins.
The main disadvantage of this catalyst is that it initiates slowly than Grubb’s 2nd generation catalyst.
Schrock’s catalyst
Schrock was able to construct a molybdenum alkylidene catalyst in 1990, which is known as schrock’s catalyst. The structure of Schrock’s catalyst is shown below:
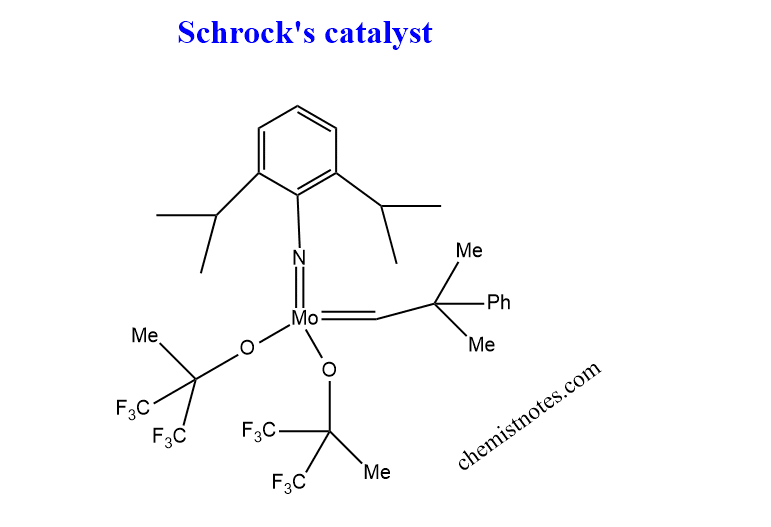
Advantages of Schrock’s catalyst
- It has high activity
- It is applicable to both terminal and internal alkene.
- Low strain cyclic olefin can undergo a ring-opening reaction using this catalyst.
- Metathesis reactions are effective even in the presence of phosphines, thioethers, and amines.
- Olefins with sterically demanding and electron-poor substituents also undergo ring-closing metathesis.
Disadvantages of Schrock’s catalyst
- It shows poor functional group tolerance even to aldehyde, acids, and alcohol.
- This catalyst is very sensitive to O2 and moisture.
- Due to its high reactivity, an inert atmosphere and dry degassed solvents are required for handling and for conducting reactions.
Let’s have an example of a reaction catalyzed by Schrock’s catalyst.
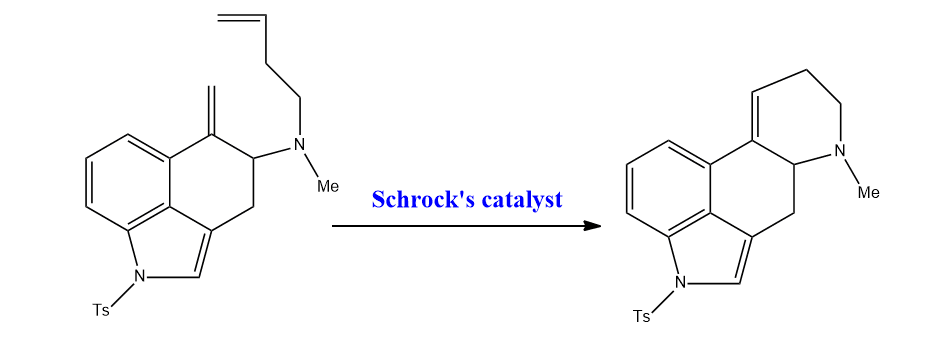
FAQs:
What is the application of Grubb and schrock’s catalysts?
These are important in organic synthesis, metathesis reactions such as ring-opening, ring-closing, ADMET, and many more synthetically important reactions.






