Table of Contents
ToggleHydrogen bond is a special strong kind of dipole-dipole attraction between molecules. Such electrostatic interaction results from hydrogen atoms covalently bonded to a highly electronegative atom (O, N, or F), and another atom that is close to the hydrogen atom and is very electronegative. Hydrogen bonds are typically stronger than dipole-dipole and dispersion forces, but weaker than covalent and ionic bonds. Its strength ranges from 4 kJ to 50 kJ per mole of hydrogen bonds.
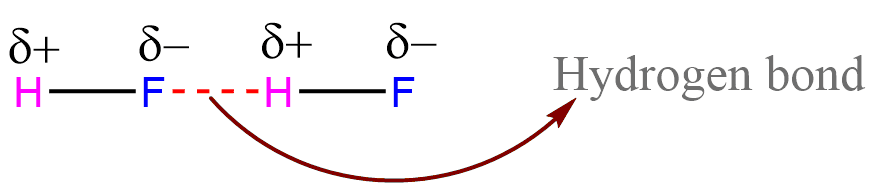
Hydrogen bond is represented by a dashed (broken line) or dotted line.
What is Hydrogen Bonding?
The term “hydrogen bonding” refers to the process of forming hydrogen bonds, which are a special type of attractive intermolecular force. Hydrogen bonds are formed as a result of a dipole-dipole interaction between a hydrogen atom that is bonded to a highly electronegative atom and another highly electronegative atom that is located in close proximity to the hydrogen atom. These dipole-dipole interactions lead to the formation of hydrogen bonds.
In water molecules (H2O), the hydrogen atom is connected to the electronegative oxygen atom via a covalent bond. As a result of the significant difference between the electronegativity of oxygen and hydrogen, the location of the bond pair of electrons in the O-H bond is situated close to the nucleus of the oxygen atom. As a consequence of this, the oxygen atom will acquire a partial negative charge (-δ), while the hydrogen atom will acquire a partial positive charge (+δ).
Now, the formation of hydrogen bonds is possible because of the electrostatic attraction that exists between the oxygen atom of one water molecule and the hydrogen atom of another water molecule. Hydrogen has a positive charge, whereas oxygen has a negative charge. Therefore, hydrogen bonds are special intermolecular attractive forces that only appear in compounds that contain hydrogen atoms that are connected to an atom of high electronegativity.
Why do Hydrogen Bonds Occur?
The very electronegative nature of the hydrogen donor (N, O, or F) causes it to draw the pair of electrons that are covalently bound to its nucleus closer to itself and further away from the hydrogen atom. After this, the hydrogen atom will have a partial positive charge, which will result in the formation of a dipole-dipole attraction between the hydrogen atom that is linked to the donor and the lone electron pair that is present in the acceptor. This leads to the formation of a hydrogen bond.
Conditions of Hydrogen Bonding
- The hydrogen atom in the molecule must be covalently bonded to a strongly electronegative atom.
- The electronegative atom should have a relatively small size. Electrostatic attraction increases as size decreases.
Characteristics of Hydrogen Bonding
- Only O, N, and F which have very high electronegativity and small atomic size, are capable of forming hydrogen bonds.
- Hydrogen bond is longer and much weaker than a normal covalent bond. Hydrogen bond energy is about 5 kcal/mole, while that of a covalent bond is about 50-100 kcal/mole.
- Hydrogen bonding results in long chains or clusters of a large number of “associated” molecules like as many tiny magnets.
- Like a covalent bond, a hydrogen bond has a preferred bonding direction. This is attributed to the fact that hydrogen bonding occurs through p-orbitals which contains the lone pair of electron on the X atom. This implies that the atoms, X-H…..X will be in a straight line.
Properties of Hydrogen Bonding
- Solubility: Because of the hydrogen bonding that can occur between water molecules and alcohol molecules, lower alcohols are soluble in water. Higher alcohols, on the other hand, are insoluble in water.
- Volatility: Because the compounds that include hydrogen bonding between different molecules have a greater boiling point, they are less volatile than other types of compounds.
- Boiling point and melting point: Hydrogen-bonded compounds typically have very high melting and boiling points. Because of the fact that hydrogen bond is comparatively stronger and additional energy is required to break hydrogen bonds, compounds with these bonds have high melting and boiling points.
- Surface tension and viscosity: Compounds possessing hydrogen bonding exist into associated/Diemer molecules resulting in difficulties in their flows. Hence, the surface tension and viscosity are comparatively higher.
Effects of Hydrogen Bonding
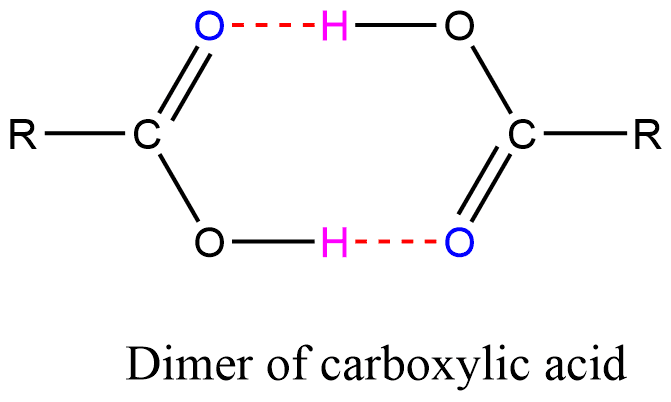
- Association: As a result of hydrogen bonding, carboxylic acid molecules can be found in the form of dimers. These kinds of compounds have molecular weights that are twice as large as those that are determined based on their common formula.
- Dissociation: When dissolved in water, HF undergoes dissociation, which results in the formation of the difluoride ion rather than the fluoride ion. This is because HF possesses hydrogen bonding properties. There is no formation of a hydrogen bond between the molecules of HCl, HBr, or HI. This provides an explanation for why chemicals such as KHCl2, KHBr2, and KHI2 do not exist.
Types of Hydrogen Bonding
Intramolecular Hydrogen Bonding
When hydrogen is bonded to two atoms of the same molecule, it is called intramolecular hydrogen bonding. It occurs within the same molecule. In order for this to take place, it is necessary for there to be both a hydrogen donor and a hydrogen acceptor present within the same molecule, and they must be located in close proximity to one another within the molecule. Some examples of intramolecular hydrogen bonding include salicylic acid, salicylaldehyde, ethylene glycol, and so on.
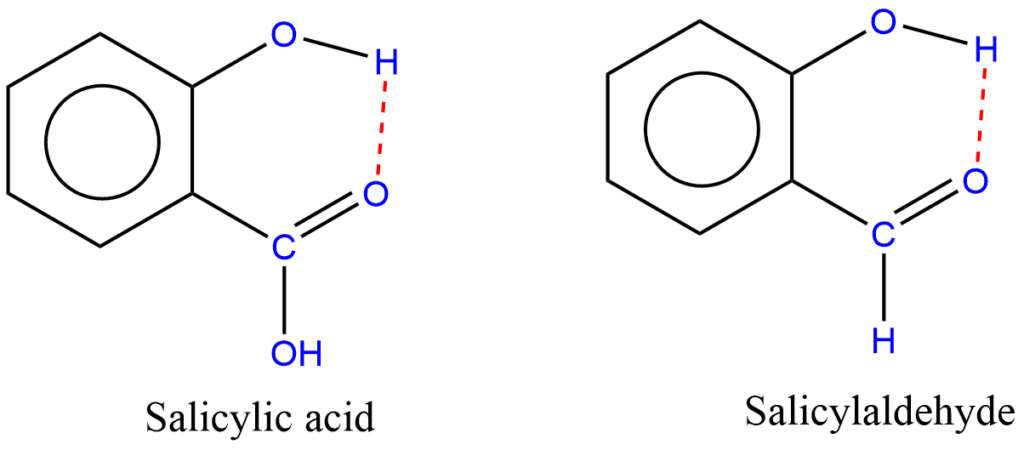
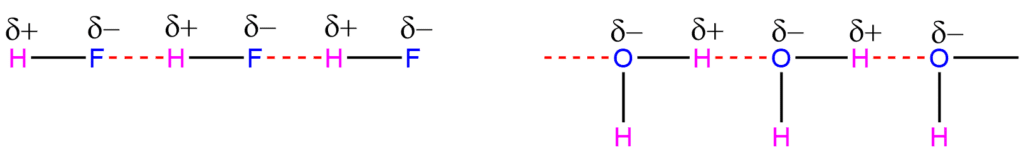
Intermolecular Hydrogen Bonding
When hydrogen bonding takes place between different molecules, it is called intermolecular hydrogen bonding. This is a special type in which two molecules can be associated as a result of hydrogen bonding. Some examples of intermolecular hydrogen bonding are HF, H2O, NH3, R-COOH, R-OH, and so on.
Strength of Hydrogen Bonding
The strength of the hydrogen bond is low. Hydrogen bonds are intermediate in strength between those of Van der Waals forces and covalent bonds. The electronegativity of an atom determines the dissociation energy of the hydrogen bond due to the attraction of the shared pair of electrons.
Examples of Hydrogen Bonding
Hydrogen Bonding in water
A highly electronegative oxygen atom is linked to a hydrogen atom in a water molecule. The shared pair of electrons are more attracted to the oxygen atom, so this end of the molecule becomes negative while the hydrogen atoms become positive.
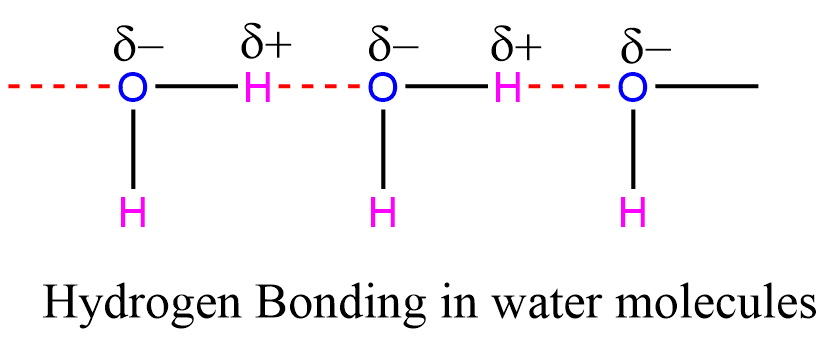
The effects of hydrogen bonds between water molecules are very important in many ways:
- Since the hydrogen bonds in ice are weaker than those in liquid water, ice can float on top of the water.
- The impact that hydrogen bonds have on the heat of vaporization contributes to the fact that animal perspiration is an efficient method for bringing the body temperature down.
- Because of its effect on heat capacity, water acts as a barrier against the rapid occurrence of temperature changes in situations that are particularly humid or contain huge quantities of water. The presence of water contributes to the stabilization of temperatures on a global scale.
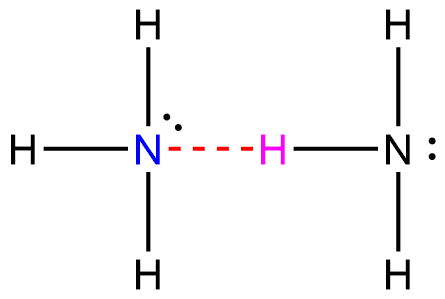
Hydrogen bonding in Ammonia
There is a formation of bonds known as hydrogen bonds between the hydrogen in one molecule and the nitrogen in another. Because each nitrogen atom only possesses a single pair of electrons, the bond that forms between ammonia molecules is of a rather weak nature.
Hydrogen bonding in HF
In HF molecules, the hydrogen bond is formed between a hydrogen atom and a highly electronegative fluorine atom.
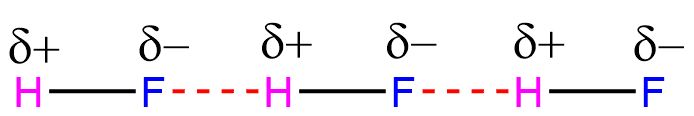
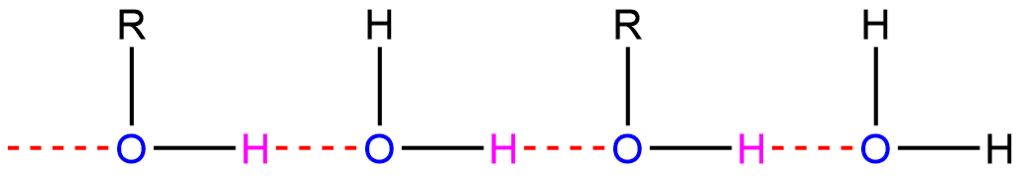
Hydrogen Bonding in Alcohol
In the case of alcohols, hydrogen bonds form between the hydrogen atoms that are partially positive and the lone pairs that are present on the oxygen atoms of other molecules.
Hydrogen bonding in Carboxylic acid
Hydrogen bonding between pure carboxylic acid molecules can result in a dimer. As a result, the van der Waals dispersion forces between any given dimer and its surroundings are increased along with the increase (almost double) in the size of the molecule, leading to a high boiling point.
Application of Hydrogen Bonding
- It explains, why H2O, HCN, etc. exist in the polymeric stage and exhibit abnormalities in boiling point, melting point, the heat of vapourization, dielectric constant, etc.
- It explains why the solvents like CHCl2F, CHCl3, etc. are more efficient solvents than similar solvents such as CCl4, CCl3F, etc.
- It plays an important role in adsorption and catalysis.
- It solves many complicated problems about the structure of inorganic compounds.
- It clearly explains why one molecule of water in CuSO4 is associated with sulfate ion and thus discloses the structure.
- The hydrogen bond plays a very important role in determining the configuration of polypeptide chains.
- The concept of hydrogen bond has been involved in explaining diverse phenomena like the chemotherapeutic action of drugs, binding of dyes in textiles, adhesive action of paints, lacquers, etc.






