Table of Contents
ToggleThe boiling point of the solvent is the temperature at which vapor pressure is equal to that of atmospheric pressure. When a non-volatile solute is added to the solvent, its vapor pressure decreases. An elevation of boiling point is the difference between the boiling points of a solution containing a non-volatile solute and the boiling point of the pure solvent.
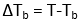
Tb = Boiling point of pure solvent
ΔTb = Elevation of boiling point
A liquid boils at a temperature when its vapor pressure equals to atmospheric pressure.
Elevation of Boiling point curve
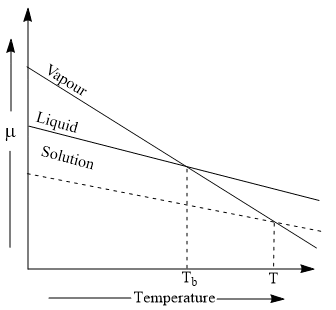
At the boiling point, the vapor-liquid curves meet and the chemical potentials are equal.
In a pure solvent at a given temperature and pressure, there exists an equilibrium between the vapor phase and the liquid phase. If the system is at 1 atm pressure, then the chemical potential of the pure component is equal to the standard chemical potential. With the addition of the non-volatile solute, the vapor pressure of the solvent will be lowered and the escaping tendency of the solvent molecule will be diminished, so more solvent molecules from the vapor phase will pass into the liquid phase to restore equilibrium.
Thus, we will have to heat the liquid to a temperature higher than the boiling point of pure liquid to increase the escaping tendency of the solvent molecules from the liquid phase.
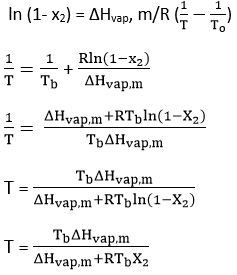
(X2) = mole fraction
This implies that with the addition of the non-volatile solute, the boiling point (T) of the solution should increase.
Molal Boiling Point Elevation
Molal boiling point elevation is the elevation in the boiling point of the solvent is caused by 1 mole of non-volatile solute dissolved per kg (1000 g) of the solvent.
ΔTb = kb × m
The proportionality constant, Kb is called the molal boiling point elevation constant. It is a constant that is equal to the change in the boiling point for a 1-molal solution of a nonvolatile molecular solute. For example, the Boiling point of water is 100 oc, when 1 mole of solute like NaCl, sugar, urea, etc is dissolved in 1 kg of water then its boiling point increases by 0.51 oc. Its value can be evaluated experimentally.
The mole fraction (X2) and the molality (m2) of the solute in a dilute solution are related by
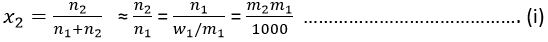
Where w1 is the mass of the solvent expressed in gram and m1 is its molar mass.

ΔHvap,m = molar heat of vaporization of the solvent from the solution
So, from equations (i) and (ii)
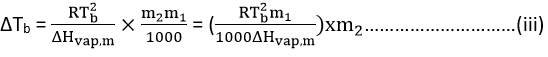
The term within parenthesis on RHS of equation iii includes the parameters of a pure solvent and hence its characteristic of that substance. Let it be represented by Kb and defined as,

ΔTb = kb × m2 = kbm…………………..(v)
Where m is unity, ΔTb = kb = molal boiling elevation
unit of kb= kmolal-1
Molal boiling point elevation of some compound
| Solvent | Normal boiling point (oc ) | molal boiling point elevation constant,kb(oc/m) |
| Acetic acid | 111.9 | 3.07 |
| Camphor | 207.4 | 5.61 |
| Naphthalene | 217.7 | 5.80 |
| Phenol | 181.8 | 3.60 |
| Water | 100.0 | 0.512 |
Molecular Weight Determination
The molar mass of the involatile solute can be calculated if we know the elevation of boiling point (ΔTb) caused by the mass of the solute dissolved in a mass of the solvent, whose elevation constant kb.
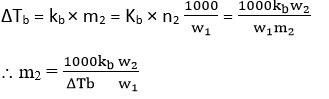
w2 = mass of solute, w1= mass of solvent.
Boiling points of some compounds
| Compound | Boiling point | Compound | Boiling point |
| Water | 100oC | HCl | – 82.05oC / 188.1K |
| Methanol | 64.7oC | HBr | – 66oC / 207.1K |
| Ethanol | 78.37oC | HF | 19.5oC |
| Acetone | 56oC | NH3 | – 33.34oC / 239.8K |
| Toluene | 100.6oC | SiH4 | – 112oC / 161.1K |
| Alcohol | 78.37oC | Milk | 100.5oC |
| Benzene | 80.1oC | CO2 | – 78.46oC / 194.7K |
| Cyclohexane | 80.75oC | NaCl | 1465oC |
| Diethyl ether | 34.6oC | NaHCO3 | 851oC |
| Ethylene glycol | 197oC | Dichloromethane | 39.6oC |
| Isopropyl alcohol | 82.5oC | Iodine | 184.3oC |
| Benzoic acid | 249.2oC | Helium | – 268.9oC / 4.22K |
| Sulphuric acid | 337oC | Argon | – 185.8oC / 87.3K |
| Acetic acid | 118oC | Oil | 300oC |
| Methane | – 161.6oC / 111.5K | Oxygen | – 183oC / 90.19K |
| Ethane | – 89oC / 184.1K | Hydrogen | – 252.9oC / 20.28K |
| Propane | – 42oC / 231.1K | Nitrogen | – 195.8oC / 77.36K |
| Butane | – 1oC / 272.2K | Fluorine | – 188.1oC / 85.04K |
| Pentane | 36.2oC | Chlorine | – 34.04oC / 239.1K |
| Hexane | 69oC | Br2 | 58.8oC |
| Heptane | 98.42oC | CCl4 | 76.72oC |
| Octane | 125.6oC | H2S | – 60oC / 213K |
| Ethyl acetate | 77.1oC | CH3OCH3 | 24oC / 249K |
| Diethyl ether | 34.6oC | CO | – 191.5oC / 81.65K |
| 1-butanol | 117.7oC | NaOH | 1388oC |
| 1-propanol | 97oC | Cyclohexane | 80.75oC |
FAQs
What is the elevation of boiling point?
The difference between the boiling points of a solution containing a non-volatile solute and the boiling point of the pure solvent is called the elevation of boiling point.
What is molal boiling point elevation?
Molal boiling point elevation is the elevation in the boiling point of the solvent is caused by 1 mole of non-volatile solute dissolved per kg (1000 g) of the solvent.






