Table of Contents
TogglePyrolytic elimination is a type of elimination reaction that takes place in presence of heat without any reagent. This reaction’s examples, mechanism, evidence, and orientation have been discussed in this post.
Pyrolytic elimination
Several kinds of compounds are eliminated when heated in the absence of any additional reagent. These kinds of reactions are known as pyrolytic eliminations. These reactions are frequently carried out in the gaseous state. Pyrolytic elimination does not require the use of an external base or solvent. This reaction is represented by Ei.
In normal elimination reactions such as E1 and E2 reactions, solvent and base are required respectively to carry out the reaction but in pyrolytic elimination, there is no requirement of such base or any other solvent to initiate the reaction.
Mostly carboxylic esters and xanthates undergo this type of elimination. Pyrolysis of xanthate ester to give alkene is a famous pyrolytic eliminations reaction, known as Chagaev elimination.
Pyrolytic elimination mechanism
Two mechanisms have been found to operate in this elimination viz pericyclic mechanism and free radical mechanism.
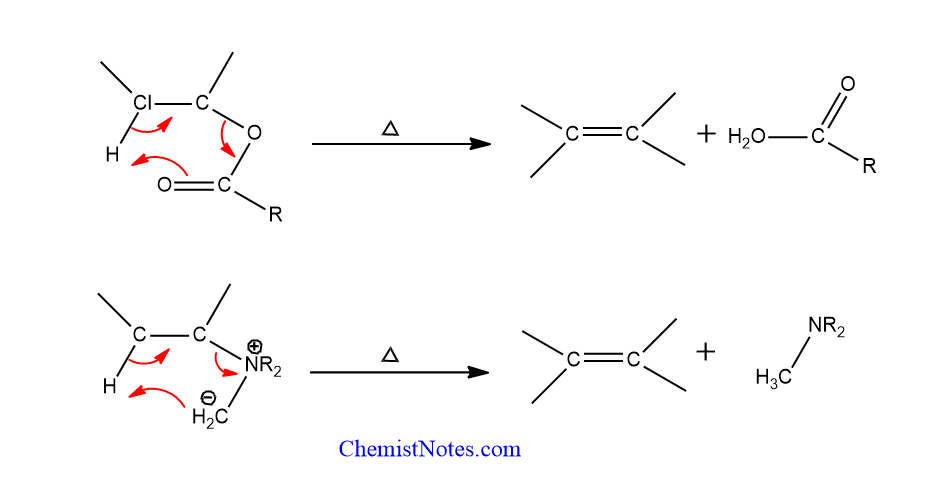
Pericyclic mechanism
This mechanism involves the formation of a cyclic transition state which maybe four, five or six-membered. In this process, two groups leave at roughly the same time and bond as they do so.
Free radical mechanism
The second type of pyrolysis mechanism is completely different and involves free radicals. Pyrolytic hemolytic cleavage initiates the process. The subsequent stages may differ, and just a few are illustrated.
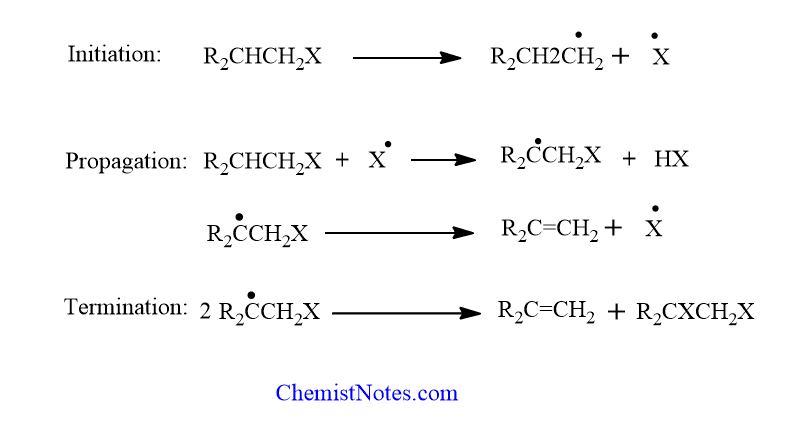
Free radical mechanisms are most commonly seen in the pyrolysis of polyhalides and primary monohalides, but they have also been proposed in the pyrolysis of certain carboxylic esters. Eliminations of free radicals in solutions are also known, but they are uncommon.
The elimination must be syn, and the four or five atoms that make up the ring must be coplanar for the four and five-membered transition states. The six-membered transition stage does not need coplanarity.
Evidence of pyrolytic elimination
Some of the pyrolytic elimination are listed below:
- Because the kinetics are first order, only one substrate molecule is involved in the process.
- Free radical inhibitors don’t slow the reactions. So, no free radical mechanism is involved.
- Exclusive syn eliminations are predicted by the mechanism.
Orientation in pyrolytic elimination
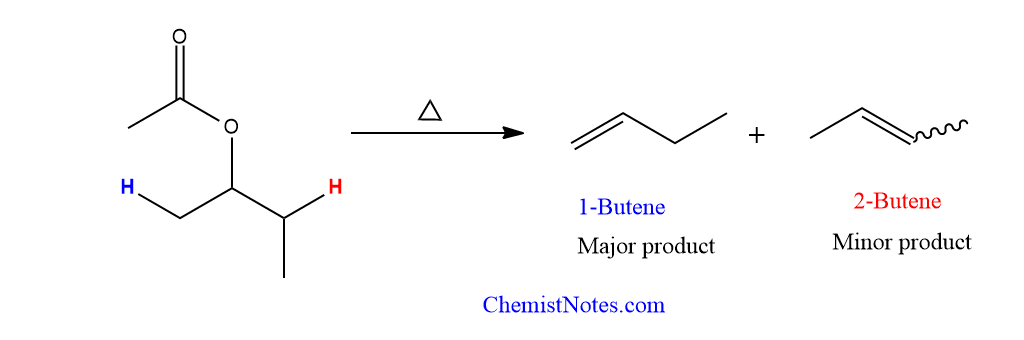
They usually follow Hoffman’s rule and remove a beta-hydrogen from the least substituted site, resulting in a less substituted alkene. Steric effects, conjugation, and stability of the generating alkene are all variables that influence product composition.
The number of beta-hydrogen available determines the orientation, hence Hofmann’s rule applies. This can be explained by taking the following example.
Sec-butyl acetate gives 1-butene as a major product and 2-butene as a minor product.
Pyrolytic elimination youtube video
References
J. March, Advanced Organic Chemistry, (4th Edition), John Wiley and Sons, 1992






