Table of Contents
ToggleSandmeyer reaction mechanism, examples, and applications in organic chemistry have been discussed here:
Sandmeyer reaction
In 1884, Traugott Sandmeyer, a Swiss chemist, reported this reaction during the preparation of phenylacetylene from benzenediazonium chloride and cuprous acetylide. When benzene diazonium salt is heated with cuprous chloride or cuprous bromide in presence of corresponding halogen acid from chlorobenzene and bromobenzene resulting the formation of aryl halide, the reaction is called sandmeyer reaction. This reaction can be used to replace an amino group on an aromatic ring with a variety of substituents.
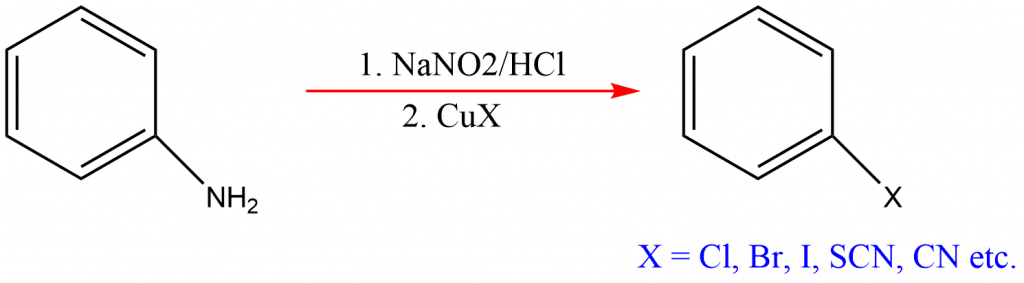
In this reaction, copper salts such as chloride, bromide, or iodide ions are used as catalysts. Since sandmeyer reaction is also used to perform transformations like Hydroxylation, trifluoromethylation, cyanation, and halogenation on benzene, it is also known as sandmeyer transformation reaction.
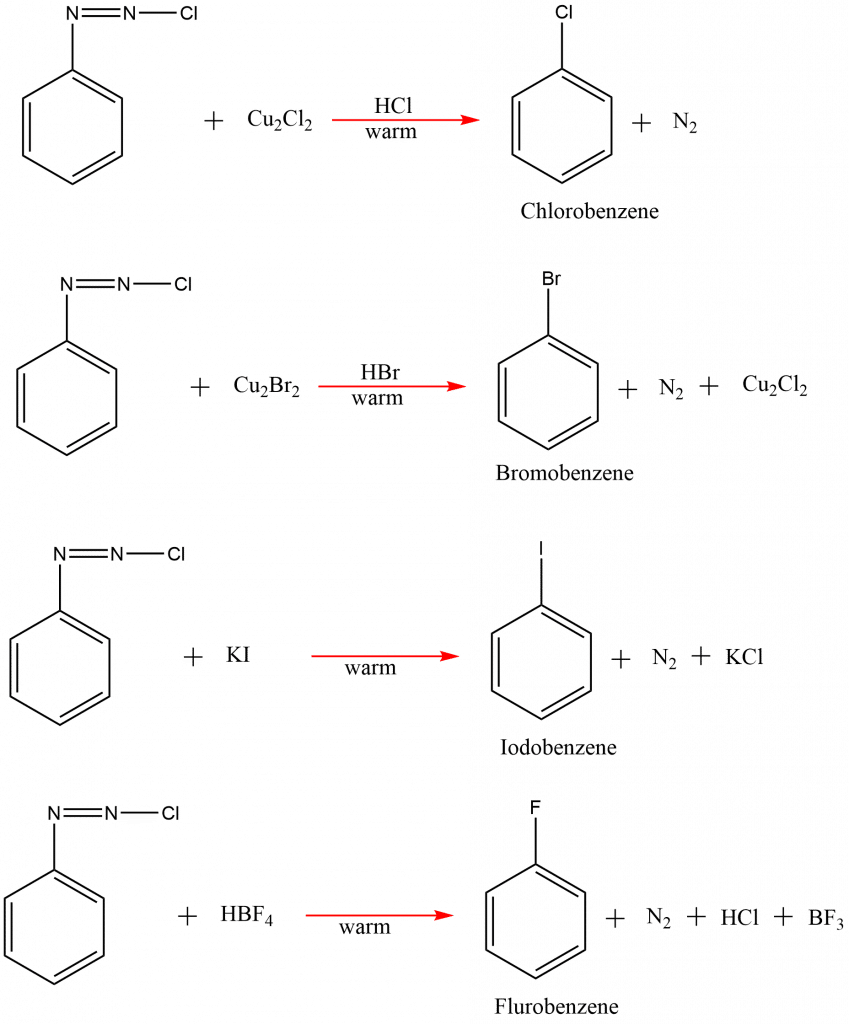
Sandmeyer reaction example
Some of the examplesof sandmeyers reactions are:
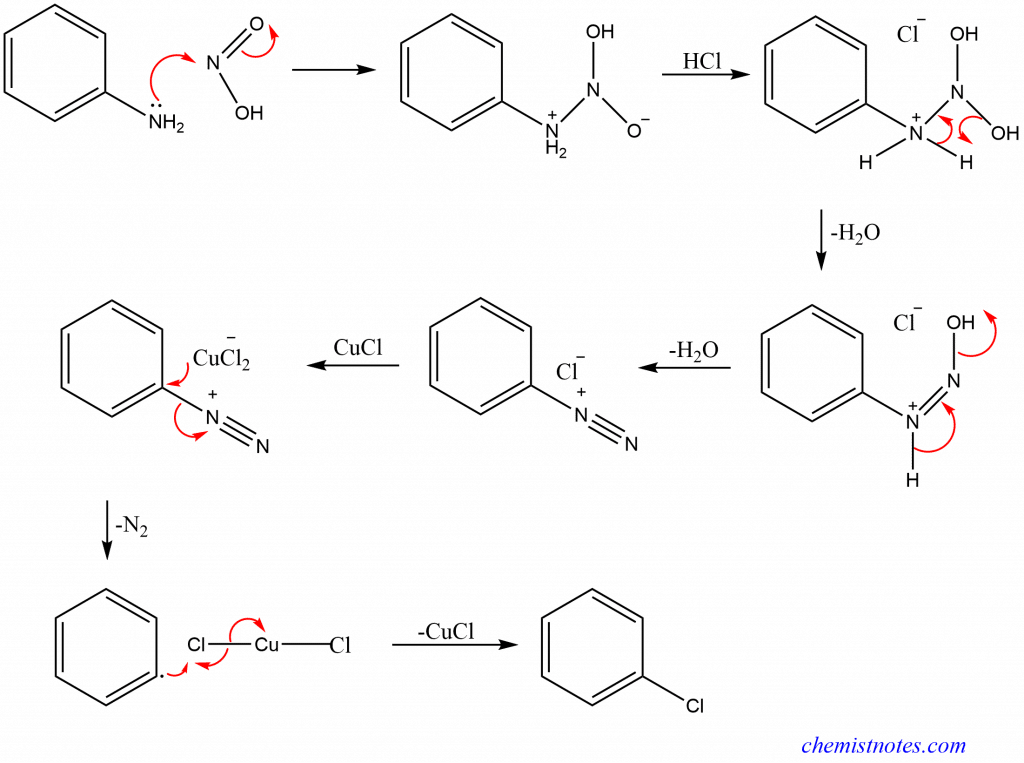
Sandmeyer reaction mechanism
The Sandmeyer reaction chemistry follows a free radical mechanism with aryl radical formation. It involves two-step synthesis of aryl halides or cyanides from primary aryl amines that involves the formation of diazo intermediates from nitrous acid followed by the transformation of diazo intermediates into aryl halides or cyanides with cuprous halides or cyanide.
The mechanism of sandmeyer reaction can be illustrated as:
Applications of sandmeyer reaction
The Sandmeyer reaction is widely used in the synthesis of aryl halides and nitriles.
Sandmeyer reaction Video
References
- Traugott Sandmeyer (1884). “Ueber die Ersetzung der Amid-gruppe durch Chlor, Brom und Cyan in den aromatischen Substanzen”. Berichte der Deutschen Chemischen Gesellschaft. 17 (4): 2650–2653. doi:10.1002/cber.188401702202
- Gehanne, K.; Lancelot, J.-C.; Lemaitre, S.; El-Kashef, H.; Rault, S. Heterocycles 2008, 75, 3015–3024.
- Suzuki, N.; Azuma, T.; Kaneko, Y.; Izawa, Y.; Tomioka, H.; Nomoto, T. J. Chem. Soc., Perkin Trans. 1 1987, 645–647.







One Response
Nice