Table of Contents
ToggleProtection and deprotection of functional groups is a very important approach in organic synthesis. The detail of this approach has been discussed in this post.
Many organic synthesis processes use substrates with multiple functional groups. If these functional groups interact or if one group reacts with a reagent in a competitive manner with another group, the synthesis may be affected.
The presence of a ketone and an aldehyde group in the same molecule, for example, may present a difficulty since they react similarly with nucleophilic species like the Grignard reagent. In the same way, having alcohol and an amine in the same molecule might produce issues.
So, what is the solution to this issue? One feasible option is to turn a reactive position or functional group into a new functional group that does not interfere with the desired transformation. Protecting groups are a type of blocking group.
The blocking of the functional groups requires at least two reactions. The first reaction transforms the interfering functional group into a different one that will not compete with the desired reaction, which is known as protection.
At a later stage of the synthesis, the second chemical step transforms the protected group back into the original group. Deprotection is the term for this.
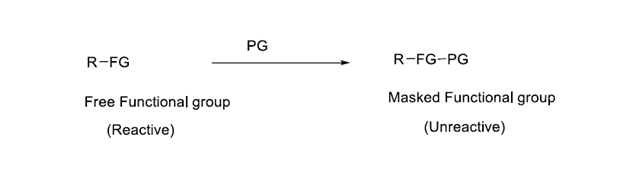
Protection and deprotection of functional groups
when a chemical reaction is to be carried out selectively at one reactive site in a multifunctional compound, other reactive sites must be temporarily blocked. This process is known as the protection of the functional group. An example of protection of the carbonyl group is shown below:
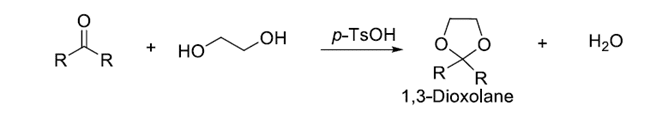
After completion of the reaction at the desired location, the protected group can be removed by suitable reactions, this process is known as deprotection of functional groups. The above-protected carbonyl group is deprotected as shown below.
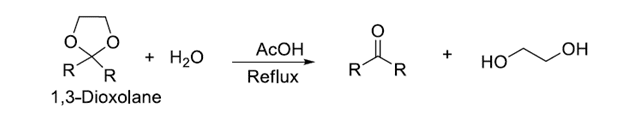
The process of protection-deprotection adds two steps to a synthesis. The protecting group must be relatively inert and ideally react with a specific reagent to unmask it.
The most important functional groups that cause reactivity problems are alcohols, ketones and aldehydes, and amines as well as carboxylic acid.
- The acidic hydrogens of the O-H groups pose issues for alcohol groups, and a protective group must remove that hydrogen. Converting the alcohol to an ester, ether, or acetal is the most typical approach for eliminating the acidic hydrogen temporarily.
- Ketones and aldehydes are subjected to nucleophilic acyl addition and thus, the carbonyl moiety is usually protected by conversion to an acetal or a ketal, a thioacetal, or a thioketal or hydrazine derivatives.
- The basic, lone pairs of electrons in an amine are the interfering portion. The lone pairs are normally not eliminated, but rather electrically delocalized via amide or sulfonamide conversion.
The following functional groups are generally protected in the chemical synthesis as discussed above.
- Protection of alcohol group
- Protection of carboxylic acid
- Protection of amine
- Protection of aldehyde and ketone
Protecting group
A protecting group is a molecular framework that is introduced onto a specific functional group in polyfunctional molecules to block its reactivity under reaction conditions.
A protecting group must fulfill a number of requirements.
- It must react selectively in a good yield to give a protected substrate that is stable to the projected reactions.
- The protective group must be selectively removed in good yield by readily available, preferably nontoxic reagents that don’t attack the regenerated functional group.
- The protective group should form derivatives without the generation of new stereogenic centers that can be easily separated from side products associated with its formation or cleavage.
- The protective group should have a minimum of additional functionality to avoid further sites of reaction.
In the end, there is no such thing as the best protective group. All of the reactants, reaction conditions, and functionalities involved in the proposed synthetic scheme should be addressed while choosing a protective group.
References
- M. B. Smith, Organic Synthesis, (3rd Edition), McGraw-Hill Companies, 1994.
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.






