Table of Contents
ToggleDuring the fragmentation process, there is a probability of intramolecular rearrangement. One such rearrangement is McLafferty Rearrangement. Although this reaction has no practical value in synthetic organic chemistry, it is one of the most studied fragmentation patterns in the field of mass spectrometry.
What is McLafferty rearrangement?
McLafferty rearrangement was first described by Fred McLafferty in 1956 and it is one of the most predictable fragmentation patterns for the ionized organic molecule in mass spectrometry.
This reaction can be defined as the rearrangement of monounsaturated molecular ion or radical ion with the cleavage of α, β-bond of the unsaturated system along with the simultaneous transfer of a γ-hydrogen via a six-membered transition state by the formation of a pair of unsaturated fragments.
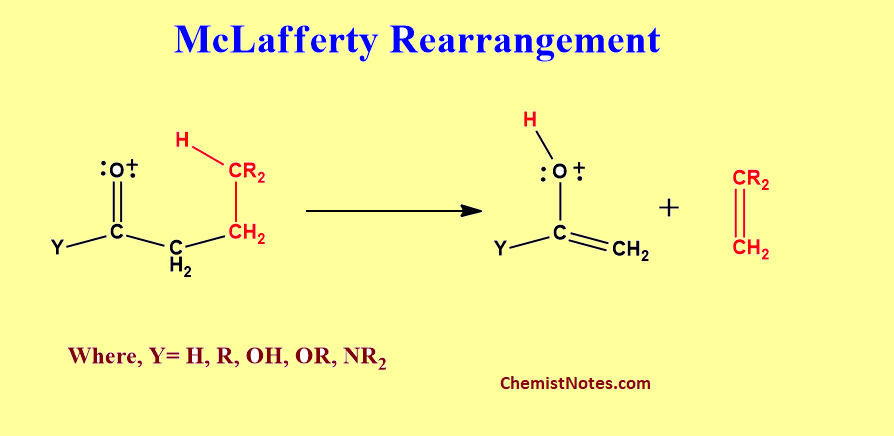
Criteria of McLafferty rearrangement
To undergo McLafferty Rearrangement, a molecule must possess the following features.
- A molecule must have suitably located heteroatoms(for example, oxygen)
- Molecules must have a π system, usually a double bond.
- There must be an abstractable γ-hydrogen atom to the C=O system or π system.
Application
This rearrangement offers a wide variety of applications in structural elucidation utilizing mass spectrometry.
McLafferty rearrangement mechanism
The McLafferty rearrangement can proceed either via a concerted process with simultaneous hydrogen transfer and β-cleavage or stepwise with the initial hydrogen transfer being followed by β-cleavage. But, experimental evidence proves that it follows a stepwise mechanism.
In this mechanism, there is the transfer of a γ-hydrogen to the oxygen of the carbonyl group, and subsequent β-Cleavage of the α,β-bond.
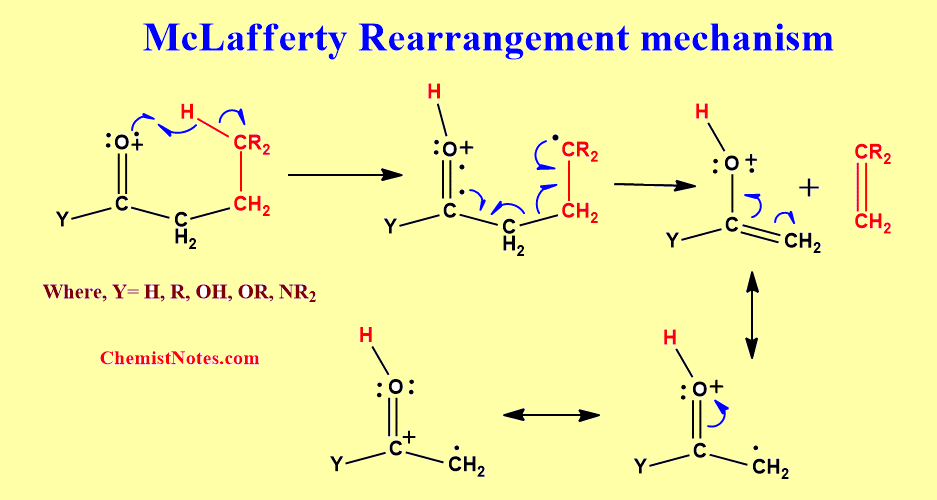
Facts about McLafferty Rearrangement
- There is usually an elimination of stable molecules during the rearrangement. Alkene is generally eliminated in this rearrangement.
- If the molecule contains a site or group having lower ionization potential than the carbonyl, no McLafferty rearrangement takes place because these groups will be ionized first. For example Aminoester, Aminoketone, etc.
- It has been reported that several factors such as structural features, and steric and electronic factors determine the actual fragmentation paths.
- It has been found that the attachment of an electron-donating group to a carbonyl group and an electron-donating group to the carbon with γ-hydrogen will enhance this rearrangement.
Misconception about McLafferty Rearrangement
We have studied that this type of rearrangement only takes place in the case of aldehydes and ketones, haven’t we?
Actually, such rearrangement takes place in many classes of compounds such as carboxylic acid, ester, amide, alkene, and alkyne as well as aromatic rings along with aldehyde and ketones. We will see later how this rearrangement takes place in the case of alkene.
McLafferty rearrangement examples
Let’s study some compounds undergoing such rearrangement with the mechanism.
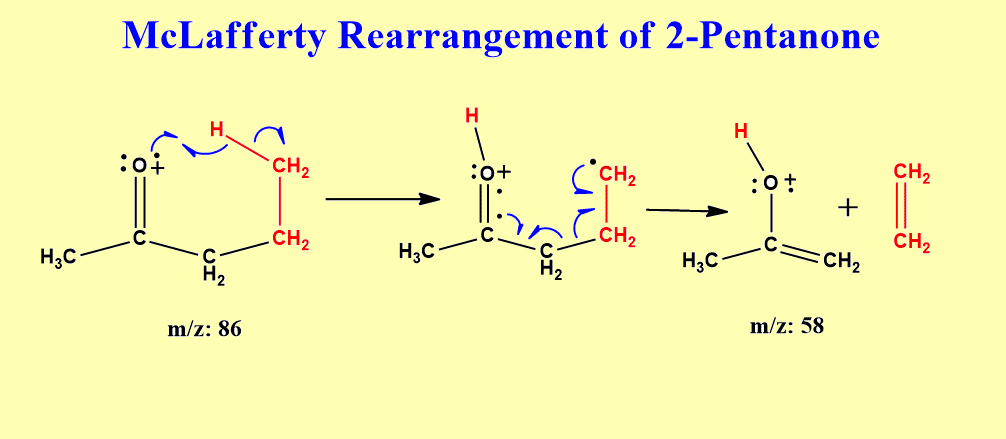
McLafferty rearrangement of 2-pentanone
2-Pentanone can undergo McLafferty rearrangement, let’s illustrate it with the mechanism.
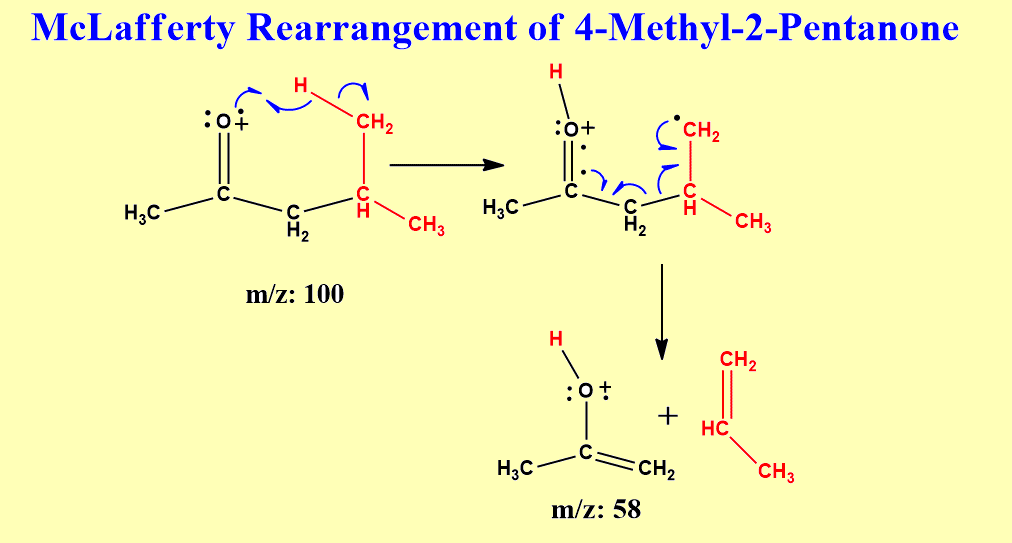
McLafferty rearrangement of 4-methyl-2-pentanone
When 4-methyl-2-pentanone undergoes McLafferty rearrangement, the propene molecule is eliminated and the resulting ion with m/z value is formed. Let’s see the mechanism.
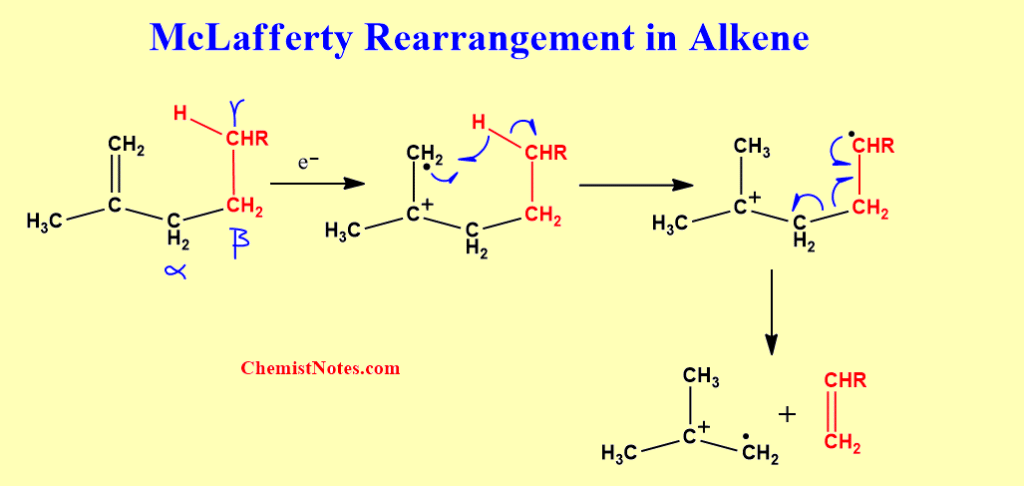
McLafferty rearrangement in Alkene
As mentioned earlier, a McLafferty rearrangement may occur in alkene provided that the γ-carbon has hydrogen on it.
Double McLafferty rearrangement
If ketones or esters having two side chains contain at least one gamma-hydrogen on each chain, such molecules can undergo two subsequent McLafferty rearrangements one after another. This is known as Double McLafferty rearrangement.
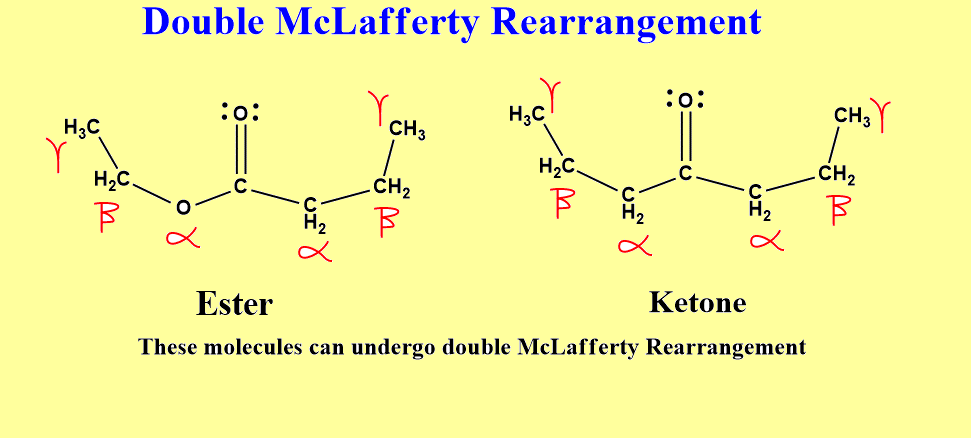
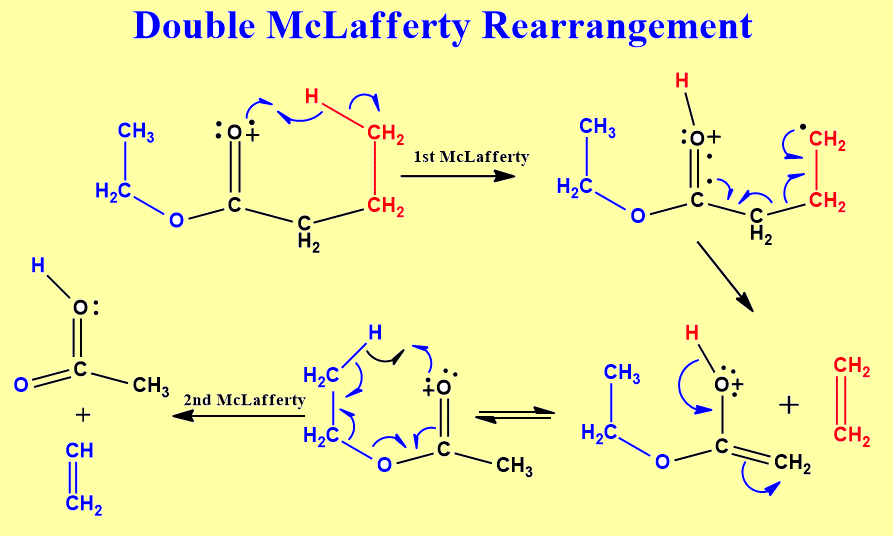
In the case of ketone, the acyl carbon atom must contain 3 more carbon atoms on either chain to undergo rearrangement. Similarly, in the case of ester, the alkoxy chain must contain 2-carbon atoms and the acyl chain must contain at least 3 more carbon atoms. This is not enough criteria to undergo rearrangement, each gamma carbon of each side must contain at least one hydrogen atom. Let’s see a mechanism to illustrate how double McLafferty rearrangement takes place.
McLafferty Rearrangement Video
References:
- https://en.wikipedia.org/wiki/Fred_McLafferty
- Jürgen H. Gross. Fragmentation of Organic Ions and Interpretation of EI Mass Spectra. 2017, 325-437. https://doi.org/10.1007/978-3-319-54398-7_6
- YunFeng Chai, YuanJiang Pan. The effect of cation size (H+, Li+, Na+, and K+) on McLafferty-type rearrangement of even-electron ions in mass spectrometry. Science China Chemistry 2014, 57 (5) , 662-668. https://doi.org/10.1007/s11426-014-5085-z
- David G. I. Kingston, Joan T. Bursey, and Maurice M. Bursey. Intramolecular hydrogen transfer in mass spectra. II. McLafferty rearrangement and related reactions. Chemical Reviews 1974 74 (2), 215-242.DOI: 10.1021/cr60288a004






