Table of Contents
ToggleThe Nuclear Overhauser effect(NOE) is a “through space” effect that can be observed by irradiating a peak corresponding to a particular proton in the sample with lower power radiation than is used for decoupling. This effect is popularly known as the NOE effect in abbreviated form.
Nuclear Overhauser effect definition
The phenomenon in which when a proton peak is irradiated at its resonance frequency, there is an enhancement in the peak intensity of a proton that is close in space to that irradiated proton is known as the Nuclear Overhauser effect(NOE effect).
This effect does not depend on intervening chemical bonds or J-coupling between protons but it relies on the dipolar interaction between spins. The prerequisite for the operation of NOE is that two protons should be within 2-4 Å in space. Moreover, the magnitude of NOE is inversely proportional to the sixth power of the distance between interacting nuclei. The closer the interacting protons(nuclei) in space, the more intense the magnitude of interaction and resulting in an intense NOE effect.
If HA and HB are two protons close in space within molecules then irradiating the peak of proton HA will enhance the peak of the Proton HB. This effect is simply known as the Nuclear Overhauser effect.
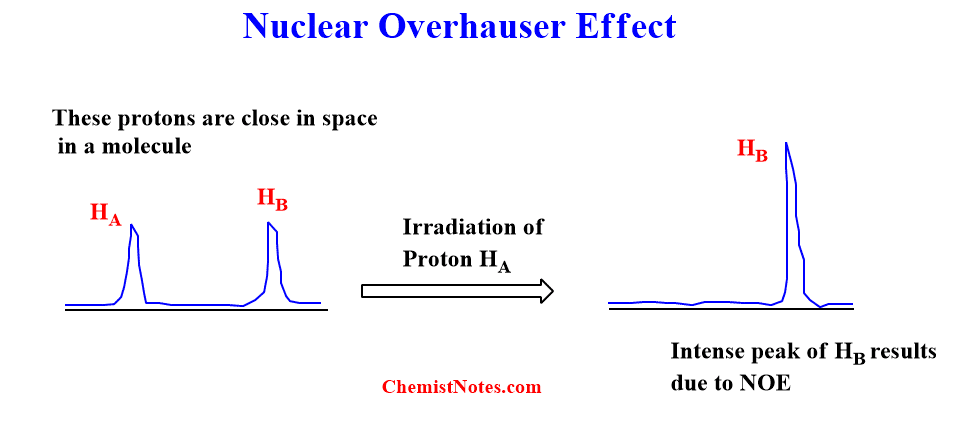
Nuclear Overhauser effect example
In the molecule given below, irradiation of the 5-methyl group resulted in the enhancement of both the H4 and H6 peaks, whereas irradiation of the 3-methyl group enhanced only the H4 peak.
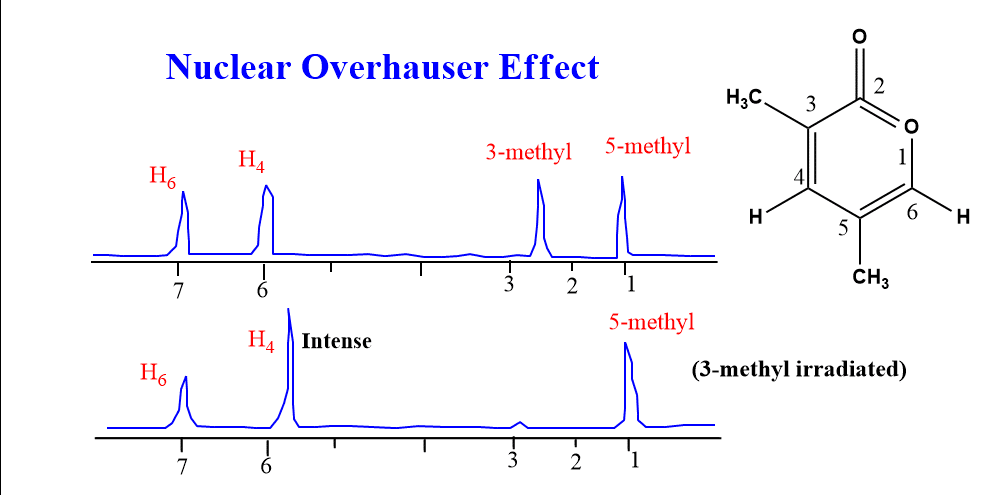
3-methyl proton is close in space with the H4 proton. Thus, when the 3-methyl proton is irradiated by applying its resonance frequency, the intensity of the H4-peak increases as shown above figure.
Similarly, when the 5-methyl proton is irradiated, the intensity of peaks of both the H4 and H6 proton enhances since these protons are close to the 5-methyl proton in the space. In a similar manner, we can find the proper position of substituents in the ring system.
Origin of Nuclear Overhauser effect
The signal enhancement in NOE is due to dipolar interaction(cross-polarization) in which the polarization of the spin states in one nucleus(proton) causes the polarization of the spin states in another nucleus. When the hydrogens in a molecule are irradiated, they are raised to an excited state from the ground state and thus they attain a situation different from the Boltzmann equilibrium distribution. In this situation, there are more spins in the excited state than in the ground state. Due to the interaction of spin dipoles in an excited state, energy transfer takes place. This causes an enhancement in the signal in the NOE effect.
Nuclear Overhauser effect applications
The NOE is an important method in NMR spectroscopy for establishing which protons are close together in space. By measuring NOE accurately, it can be used to demonstrate that two protons or groups of protons are in close proximity in space within a molecule. Therefore, it has valuable application in the structural elucidation, determination, and study of molecular geometry as well as stereochemistry.
Significance of Nuclear Overhauser effect
The significance of Nuclear Overhauser effects can be understood by reading the following points.
- During NMR spectra interpretation, the spatial location of protons within a molecule may not be known completely. In such cases, the NOE experiment is very useful to distinguish the protons by their spatial locations. For example, the groups are either cis to each other or trans in alkenes can be determined.
- In many bicyclic compounds, organic chemists may wish to determine whether the substituents are in exo or endo position. Many such types of problems can not be solved by analysis of chemical shifts or by the spin-spin coupling phenomenon. To solve these problems, NOE difference spectroscopy is very useful.
- In the NOE difference experiment, a conventional 1H-NMR peak is subtracted from a specific proton irradiated spectrum. Thus, we obtain a spectrum having only the peaks that are enhanced due to the NOE effect.
Not only NOE difference experiment, to increase accuracy in the structural elucidation of complex molecules like natural products there is 2d-NMR spectroscopy based on this NOE, popularly known as NOESY spectroscopic technique.
FAQs/MCQs:
Why should the NOE experiments be carried out in deoxygenated samples?
Since molecular oxygen is paramagnetic, it can contribute to the nuclear spin-lattice relaxation processes. To avoid the interference of oxygen with the NOE experiment, it should be carried out in deoxygenated samples.
NOE video






