The Kolbe reaction is a type of addition reaction also known as the Kolbe-Schmitt reaction. When sodium phenoxide is heated with carbon dioxide at about 125oC and under 4 to 7 atmospheric pressure, sodium salicylate is formed as a major product. This on acidification gives salicylic acid. This reaction involves the carbonation of phenol to give phenolic acid and is called the Kolbe reaction.
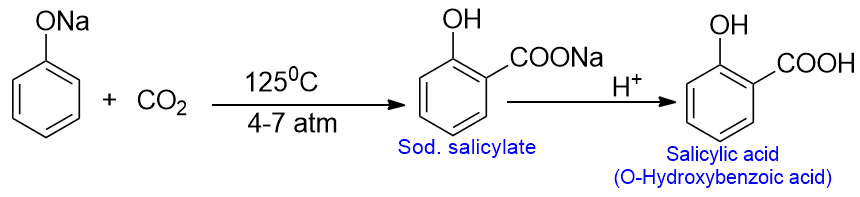
Although a small amount of para isomer is also formed, they can be separated by means of stem distillation, as the ortho isomer is steam volatile.
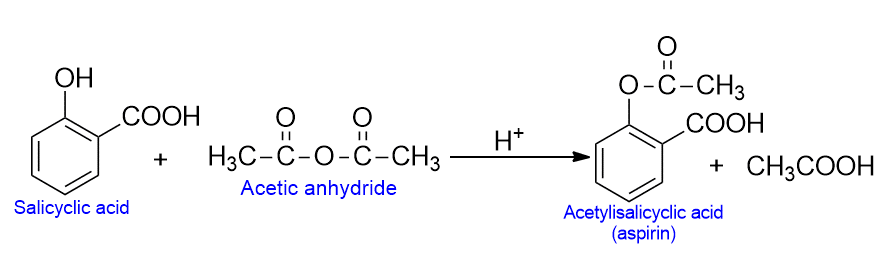
A number of important compounds can be manufactured from salicylic acid. For example, the reaction of salicylic acid with acetic anhydride and conc. H2SO4 yields the widely used analgesic called aspirin.
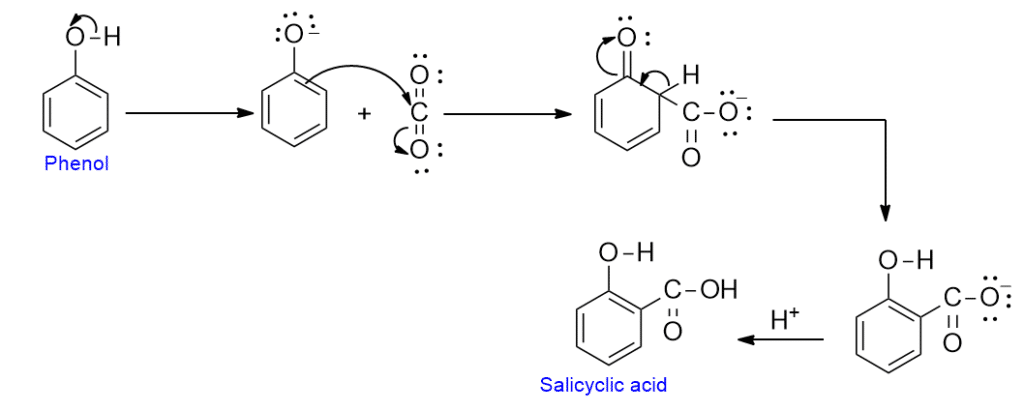
Mechanism of Kolbe reaction
In this reaction, CO2 initially attaches itself to phenoxide oxygen rather than to the ring. In any case, the final product almost certainly results from the electrophilic attacks by electron-deficient carbon on the highly reactive phenoxide ring.
;; Read: reactions of phenol
Application of Kolbe reaction
- A number of important compounds can be manufactured from salicylic acid, The reaction of salicylic with acetic anhydride and conc. H2SO4 yields the widely used analgesic called aspirin. Aspirin is a commonly used painkiller.
- The Kolbe reaction is also applicable to the industrial synthesis of 3-hydroxy-2-napthaoic acid, which is a common source of azo dye and pigment.
- If potassium hydroxide is used in the Kolbe reaction, 4- hydroxybenzoic acid can be obtained, which is a key precursor for parabens, used as a biocide in cosmetic products.






