Table of Contents
ToggleTo read an NMR spectrum of an organic compound, we must have basic knowledge about nuclear magnetic resonance spectroscopy. Do you know how the NMR spectra are generated? If not please visit our post on NMR spectroscopy.
How to read NMR spectra?
Ok, let’s move to our topic “how to read NMR spectra?”. We have tried to explain some basic steps to analyze the 1H-NMR spectra of any compound.
Step 1: Determine number of signals in the spectra
By observing signals in the spectra, we can tell the number of different types of hydrogen present in the compound. Simply, the number of signals is equal to the number of non-equivalent hydrogen in the compounds. Let’s illustrate by considering the following example. Suppose we have a proton-Nmr spectrum as shown below.
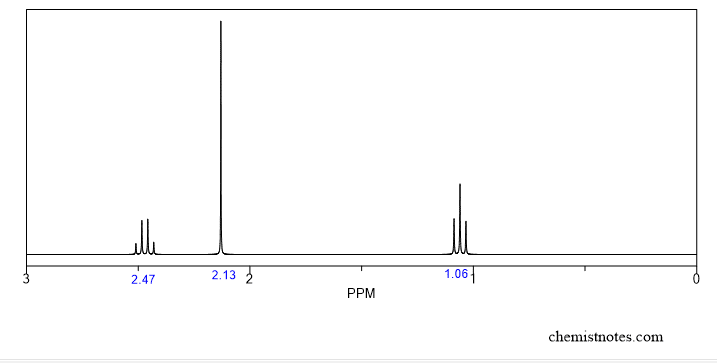
In this spectrum, we can see three different signals which indicate that there are 3 types of non-equivalent hydrogens in the compound.
Step 2: Determine the chemical shift value of each signal
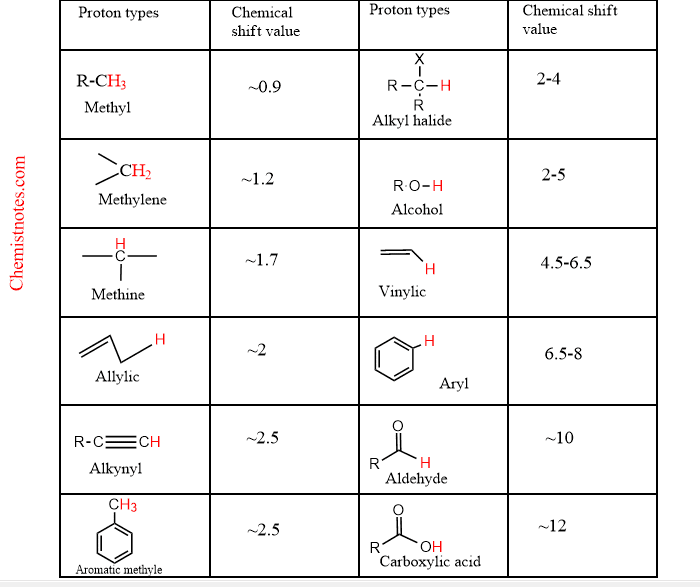
The chemical shift value of the signal gives an idea about what types of protons are present in the compounds. Moreover, the chemical shift value gives information about the chemical environment of the proton. For example, the proton of aldehyde group R-CO-H has a chemical shift value in the range of 9-10 PPM. Don’t worry, you can have an idea about it by looking at the table below, which have proton types and corresponding chemical shift value.
Now, look at the above spectra and table. There are types of signals having different values of chemical shifts 1.06, 2.13, and 2.47 ppm. This chemical shift value indicates which types of proton are present in the compounds.
- Chemical shift value 1.06 indicates that there is a methyl group.
- Chemical shift value 2.13 indicates there is a proton attached with double bond.
- Chemical shift value 2.47 indicates there is may be any group(alcohol, alkyl halide, aromatic methyl etc), which will be confirmed later.
Step 3: Determine the multiplicity of signal.
After observing the chemical shift value, now look at the splitting of the signal. The signal may be singlet, doublet, triplet, quartet, and so on. The neighboring non-equivalent proton affects the signal of a proton. The proton signal splitting takes place according to (N+1) RULE. where N= number of neighboring non-equivalent protons.
Let’s see the splitting of the signal of the above spectra:
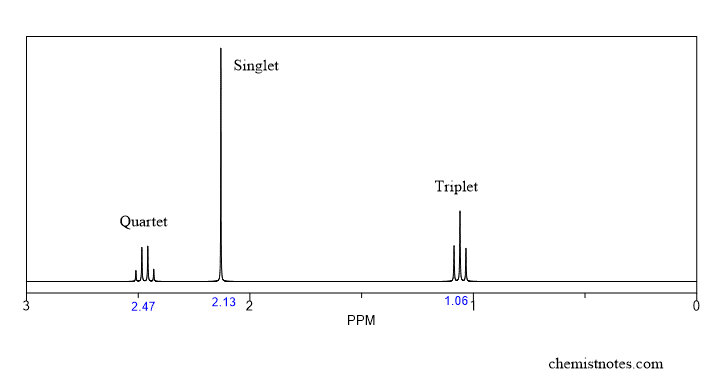
In this spectrum,
- the signal at 1.06 ppm has splitted into 3 signals. That means there are 2 neighboring protons.
- signal at 2.13 ppm has not splitted, which means there are no neighboring protons.
- signal 2.47 ppm has splitted into 4 signals, which means there are 3 neighboring protons.
Step 4: Determine integration value of each signal
The integration value gives an idea about the number of protons giving a particular signal. The computer generates integration value and the ratio of the integration values of each signal has significance. Suppose, the ratio of integration value is 1:2:1. This means one proton is responsible for giving one signal, two protons are responsible for giving 2nd signal and one proton is responsible for giving 3rd signal. This gives idea rough idea of the total number of protons in the compounds.
Step 5: Draw correct fragements of each signal.
After knowing chemical shift, integration value, no. of signal, multiplicity, just start drawing correct fragments of compounds showing particular signal. We have drawn the fragments of the above spectrum. Have a look.
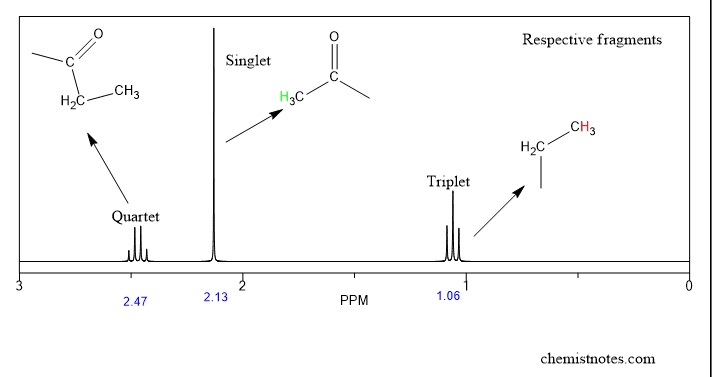
Step 6: Assemble these fragments correctly
After generating fragments, try to combine these fragments correctly to produce a molecular structure. We have combined the above fragments and the following structure obtained.
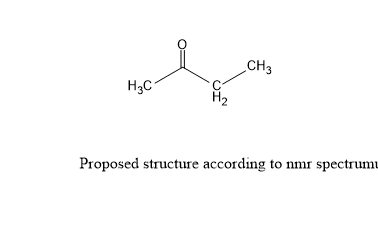
Step 7: Verify the proposed structure.
To confirm the proposed structure, compare it with standard substance or we can use ChemDraw software to generate a rough spectrum of compounds.
You can also draw a spectrum of given compounds by using these concepts. If you got confused, comment below.
Thank you. Happy learning.








2 Responses
very good explanation for NMR spectra. we are benefited very much.
Thank you very much.