Table of Contents
TogglePhenol are hydroxy derivatives of aromatic hydrocarbons in which the hydroxy group is directly attached to the carbon atom of the aromatic ring. They are represented by the general formula Ar-OH, where Ar may be phenyl, substituted phenyl, or some other aryl group like naphthyl. If the -OH group is present in the alkyl side chain, the compound is not considered a phenol. It is called aromatic alcohol as it resembles aliphatic alcohol in its characteristics.
Phenols differ from alcohols in their methods of preparation and in their chemical behavior. However, because both compounds contain the -OH group, they have some similarities.
Classification of Phenol
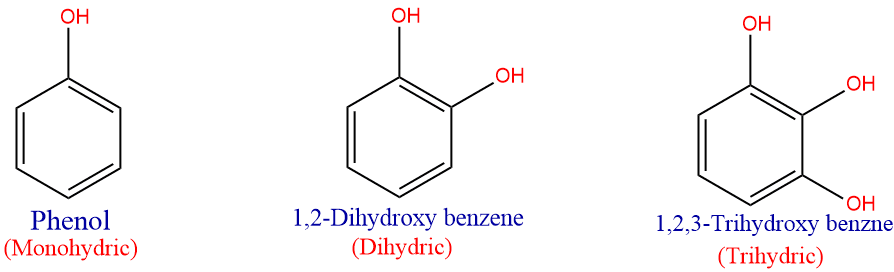
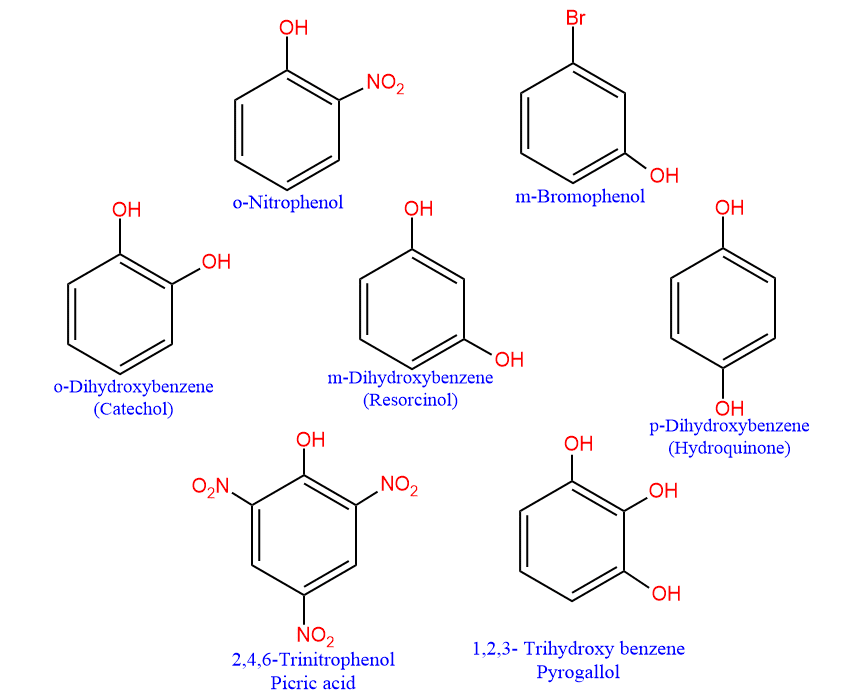
Similar to the classification of alcohol, the number of -OH groups in the aromatic rings determines whether a phenol is;
- monohydric: contain one -OH group,
- dihydric: contain two -OH group,
- trihydric: contain three -OH group
Structure of Phenol
The oxygen atom of the phenolic -OH group is in an sp2 hybridized state. It has two lone pairs of electrons, one in p-atomic orbitals and the other in an sp2 aromatic orbital; the -OH group lies on the plane of the ring. The p-atomic orbital is parallel to the p-atomic orbitals of the ring and perpendicular to the ring. Therefore, the p-electrons of the OH group undergo p-π conjugation and are delocalized over the ring. Its structure is thus supposed to be a resonance hybrid of the following canonical forms:
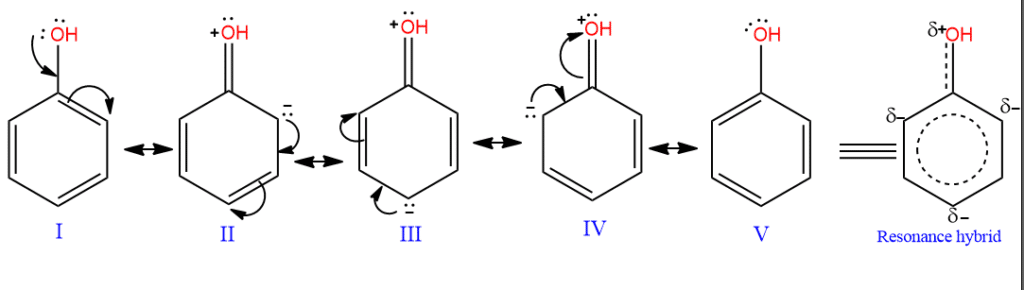
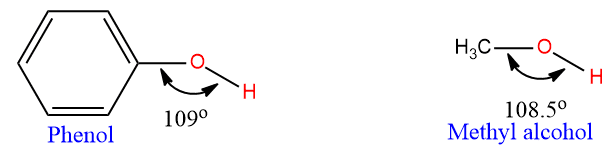
It is evident that the contribution of structures II, III, and IV imparts a partial double bond character to the C–O bond. Thus, the C–O bond in phenol (1.36 Ao) is less than the C–O bond in methyl alcohol (1.42Ao).
Nomenclature of Phenols
According to the common system of nomenclature, the parent member of the family is phenol. The other members have named them as derivatives of phenol. The prefixes ortho (O-), meta (M-), or para (P-) may be used to indicate the positions of the substituents. In the IUPAC system, numerals are used for this purpose.
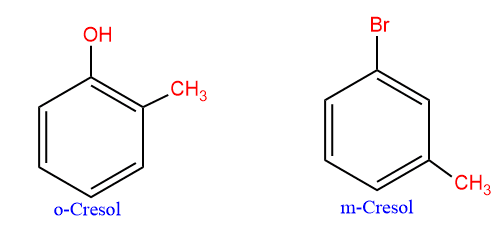
The methyl phenols are commonly called cresol.
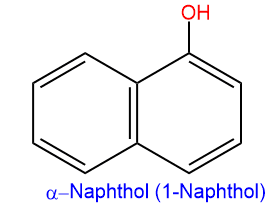
When a hydroxyl group is attached to a polycyclic benzenoid ring, then the compounds are called naphthols.
Physical Properties of Phenol
- Phenols are liquids or low-melting solids. They are colorless when pure. But many phenols are colored by oxidation products, as they are easily oxidized in air. They have characteristic phenolic odors.
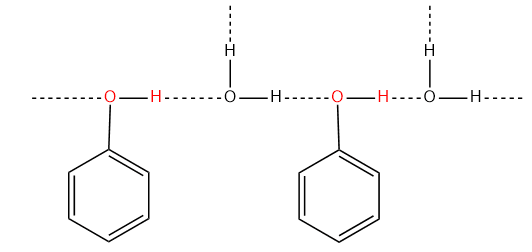
- Phenols are capable to form intermolecular hydrogen bonds, and hence they exist as associated molecules. Due to this reason, the melting and boiling points of phenols are higher than that of aromatic hydrocarbons of comparable molecular masses.
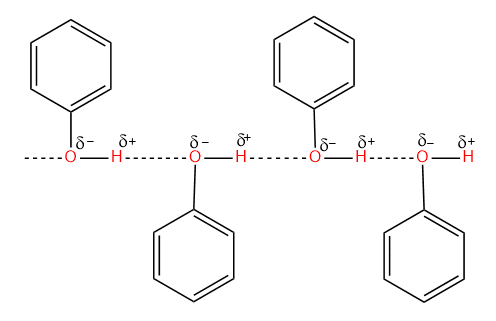
- Phenols are sparingly soluble in water (9.3 gm/100gm H2O). The solubility of phenol is due to the formation of hydrogen bonds with water. Most of the other phenols are insoluble in water.
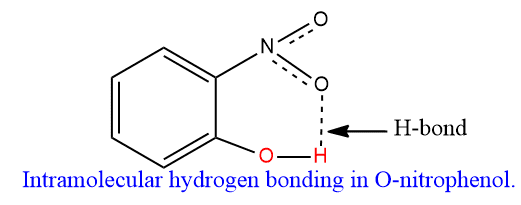
- The boiling points and solubility of phenols are affected by the presence of a substituent such as the -NO2 group. For example, when a nitro group is present in the ortho position to the phenolic group, intramolecular hydrogen bonding occurs as -NO2 and -oh groups are very close to each other. Consequently, the association among phenol molecules does not occur. Thus O-nitrophenol has a low boiling point and hence is more volatile than m- and p-nitrophenols. As it cannot form intermolecular hydrogen bonding with water, its solubility in water is very low.
- On the other hand, the m- and p-isomers of nitrophenols are capable of forming intermolecular hydrogen bonding. As a result, these isomers have very high boiling points. The m- and p- isomers of nitrophenols have higher solubility as compared to O-nitrophenol. This is also due to the intermolecular hydrogen bonding with water molecules.
Preparation of Phenol
The following method of synthesis of phenol may be used for laboratory preparation.
Hydrolysis of Diazonium salt
This is the most important laboratory synthesis of phenols and involves the hydrolysis of diazonium salts. The reaction begins with the preparation of diazonium salts by treating primary aromatic amine dissolved in cold aqueous mineral acid with sodium nitrate.
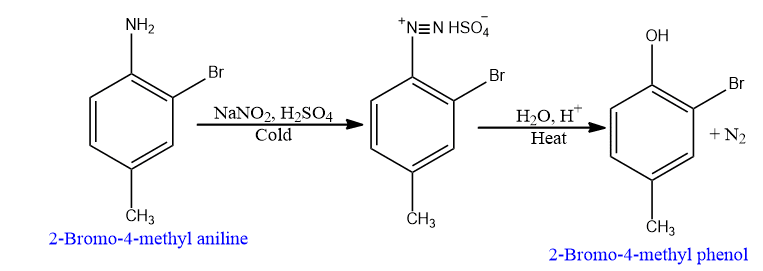
The hydrolysis of diazonium salt is a highly versatile method for the preparation of phenols. Mild conditions are required for the diazonium step (Ist step) and the hydrolysis step (IInd step), and other groups present on the ring (Br and CH3 in the above example) remain the same.
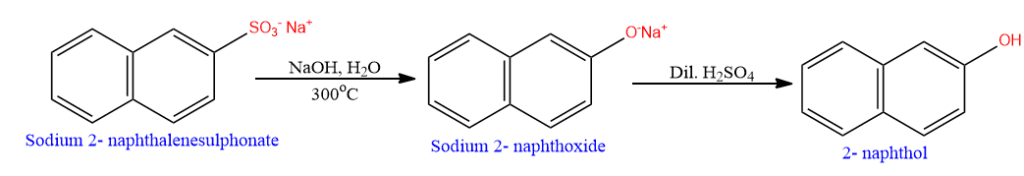
Alkali fusion of Sulphonates
Phenols can be prepared in the laboratory by the fusion of sodium arenesulphonates with sodium hydroxide at about 300oC.
Uses of phenol
- Phenol has been the most often used disinfectant, and it also has incredible antibacterial qualities.
- It is one of the most often used chemicals for cleaning domestic goods, particularly washrooms and floor tiles.
- Many compounds, including picric acid, pharmaceutical medications, explosive materials, and plastic polymers, contain phenol as a starting material.
- Phenol has been shown to be a powerful antibacterial, antifungal, and antiviral agent.
- Phenol is used to make a variety of skincare and cosmetics products such as skin-lightening creams and lotions, sunscreens, and hair color and dye solutions.
- A relatively dilute concentration of phenol can relieve a sore throat.
- Phenol was one of the materials used by Hitler in the Second World War for to execute the Nazi community due to its deadly properties when used in high concentrations.
FAQs
What is phenol used for?
Phenol has been the most often used disinfectant, and it also has incredible antibacterial qualities.
What are phenols?
Phenols are hydroxy derivatives of aromatic hydrocarbons in which the hydroxy group is directly attached to the carbon atom of the aromatic ring.






