Table of Contents
ToggleAccording to the HSAB principle, hard acid prefers to combine with a hard base and soft acid prefers to combine with a soft base in order to form a stable acid-base complex. Ralph G. Pearson proposed the HSAB concept, where hard acids interact strongly with a hard base to form stable ionic complexes, while soft acids interact strongly with a soft base to form covalent complexes. It is widely used in explaining the stability of complexes, reaction mechanisms, and their pathways. Moreover, it is also applicable in predicting the product of metathesis reactions.
HSAB Principle
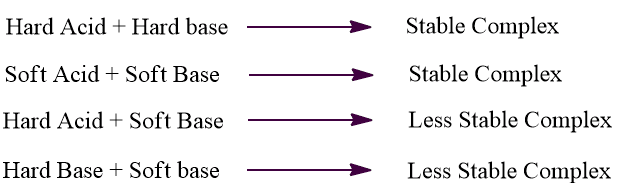
According to the HSAB principle, hard acid binds most strongly with a hard base and soft acid binds most strongly with soft bases i.e. hardness prefers hard-hard interactions and softness prefers soft-soft interactions. The combination between hard acid and soft base or hard base and soft acid will produce a less stable acid-base complex.
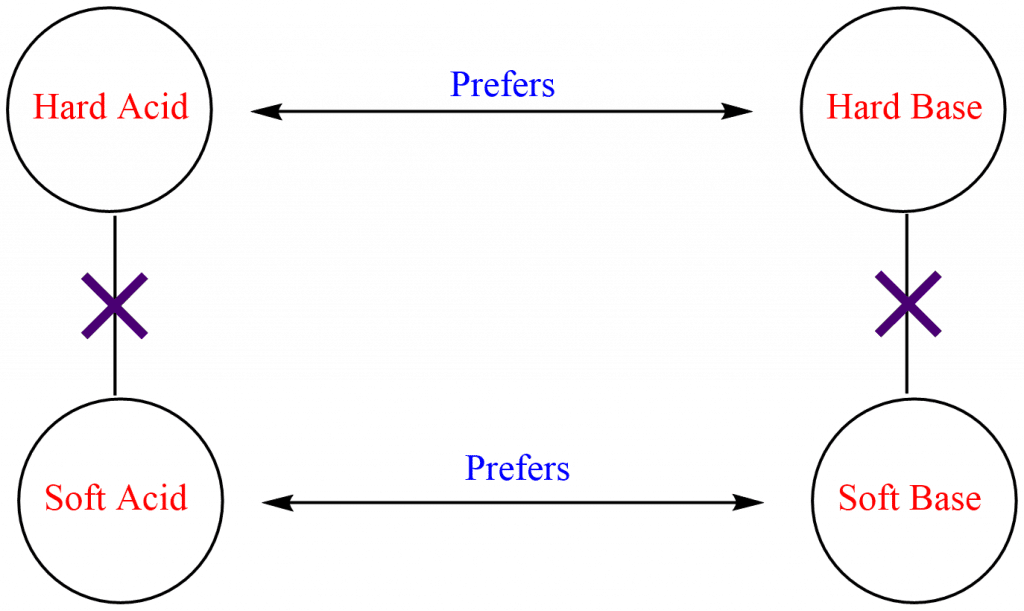
Strong ionic interaction results from a large electronegativity difference between hard acid and base, while the interaction between soft acid and base leads to strong covalent interaction. However, the interaction between hard acid and soft base or soft acid and hard base results in polar covalent interactions which are more reactive and less stable. This is the reason that hard acid prefers to combine with hard acid or soft acid prefers to combine with a soft base in order to form more stable complexes.
HSAB principle helps to predict whether the complex formed between acid and base is stable or not.
For Example:
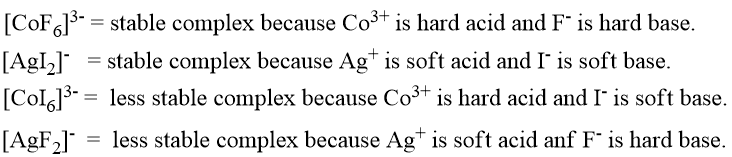
Based on the HSAB concept, Lewis acids or bases are classified into hard and soft. The hardness and softness of acids and bases depend on how firmly the electron cloud is held by any species. A firmly held electron cloud with low polarisability makes a species hard, while a loosely bound electron cloud with high polarisability makes the species soft. In general, species having relatively high electronegativities are hard, while those having low electronegativities are soft. However, the third category, which is an intermediate category, appears on the borderline.
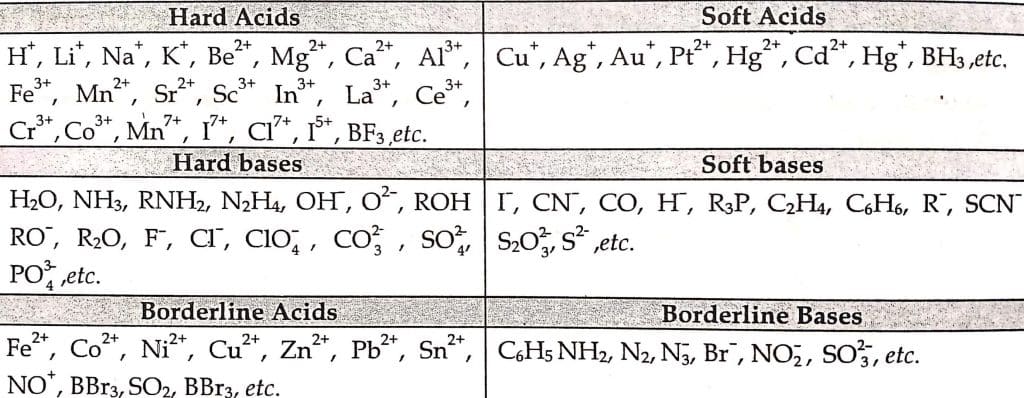
Hard acids
The acceptor atoms are small, have a high positive charge, and do not contain unshared pairs in their valence shells. They have low polarizability and high electronegativity.
For example: H+, Li+, Na+, K+, Mg2+, Ca2+, Al3+, Cr2+, Fe3+, BF3, B(OR)3, AlMe3, AlCl3, AlH3, SO3, RCO+.
Hard Bases
The donor atoms are of high electronegativity and low polarizability and are hard to oxidize. They held their valance electrons tightly.
For example H2O, OH–, F–, AcO–, SO42-, Cl–, CO32-, NO3-, ROH, RO–, R2O, NH3, RNH2
Soft Acids
The acceptor’s atoms are large, have a low positive charge, and contain unshared pair of electrons (p or d) in their valence shells. They have high polarizability and low electronegativity.
For example: Cu+, Ag+, Pd2+, Pt2+, Hg2+, BH3, GaCl3, I2, Br2, CH2, Carbenes
Soft Bases
The donor atoms are of low electronegativity and high polarizability and are easy to oxidize. They hold their valance electrons loosely.
For example R2S, RSH, RS–, I–, R3P, (RO)3P, CN–, RCN, CO, C2H4, C6H6, H–, R–
The overall classification can be illustrated as:
Theoretical Basis of Hardness and Softness
To explain the stability of complexes, different theories have been proposed, formed as a result of interactions between hard and soft elements. Some important theories are:
- π-Bonding theory: Mulliken (1955) and Chatt (1956) proposed this hypothesis to explain soft-soft interaction on the basis of π-bonding. Soft acids have a low oxidation state and have an enormous number of d-electrons. Thus, they have a strong tendency to form π-bonds with soft bases which are also good π–bonding ligands. The polarization of soft acids and soft bases even prefers π-bonding.
- Electronegativity and Hardness & softness: Elements with high electronegativity are hard and elements with low electronegativity are soft. The relation between electronegativity and hardness helps to explain the fact that CF3 is considerably harder than CH3 and BF3 is harder than BH3.
- Electrostatic Interactions: This hypothesis states that the interaction of hard acids and hard bases leads to the formation of an ionic bond. Hard-hard interactions are thus purely ionic or electrostatic. The electrostatic force of attraction between two oppositely charged ions is inversely proportional to the internuclear distance. The internuclear distance will be less in the case of smaller ions. Therefore, the electrostatic attraction between two ions will be more prominent and consequently the resulting compound formed by hard acid and hard base will be highly stable.
Application of the HSAB principle
- Alkenes and aromatic rings are soft bases and should prefer to complex with soft acids. Thus, Ag+, Pt2+, and Hg2+ complexes are common, but complexes of Na+, Mg2+, or Al3+ are rare.
- The occurrence of the ores of certain metals can be explained on the basis of the HSAB principle, for example, hard acid metal ions such as Mg2+, and Ca2+ occur mostly as their oxides, carbonates, or halides.
- HSAB principles have been applied to analyze the reactivity of ketone and ester enolate anions and to analyze catalyst selectivity in synthesis.
- The HSAB principle can also predict the formation of various metal ion complexes with ambident ligands and it is widely used to explain the stability of complexes.






