Table of Contents
ToggleMetathesis reaction is a chemical reaction that involves the exchange of one component from each of two compounds to create a new product with the aid of transition metal complexes. Two Greek terms, “meta” and “thesis”, were combined to form the term “metathesis.” Meta means change and thesis means position. Olefine metathesis or alkene metathesis are simply called metathesis reactions.
Metathesis reaction definition
Olefin metathesis is a reaction in which, in the presence of certain transition metal compounds, all of the carbon-carbon double bonds in an olefin or alkene are broken and then statistically reorganized.
Olefin metathesis is a powerful reaction that transforms carbon-carbon double bonds and, with the help of certain metathesis catalysts, new carbon-carbon double bonds are produced at or near room temperature from a substrate with a range of functional groups.
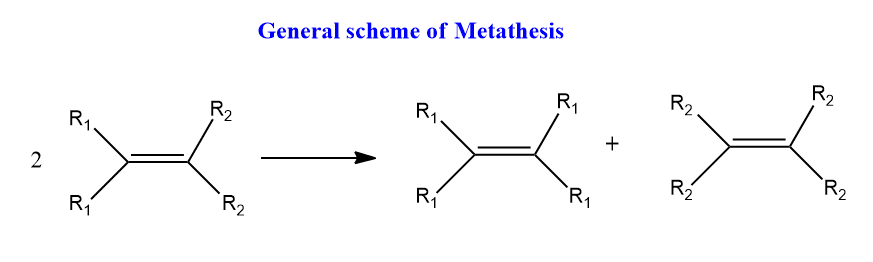
The formal interchange of substituents group attached to the C=C bond of either the same or two different alkenes is termed π-bond metathesis or olefin metathesis or simply metathesis. Alkynes can also undergo these interchange by similar pathways. Another type of metathesis which involves the reorganization of single bonds is called sigma-bond metathesis.
Olefin metathesis is a reaction catalyzed either homo or heterogeneously primarily by complexes of Ru, Mo, W, Re, and some group 4 and 5 metals. These reactions often occur under very mild conditions of temperature and pressure. Complexes of Ru, Mo, and W seem to show the most activity.
Types of metathesis reactions
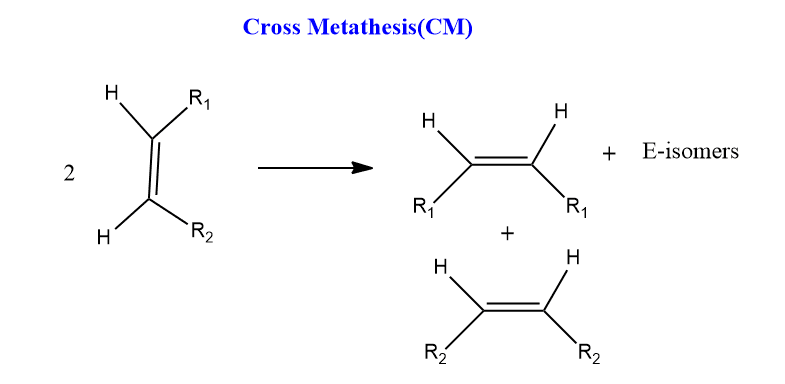
Cross Metathesis(CM)
Cross-metathesis refers to the direct exchange of groups between two alicyclic olefins. Cross-metathesis with heterogeneous catalysts is used in the industrial manufacture of olefins.
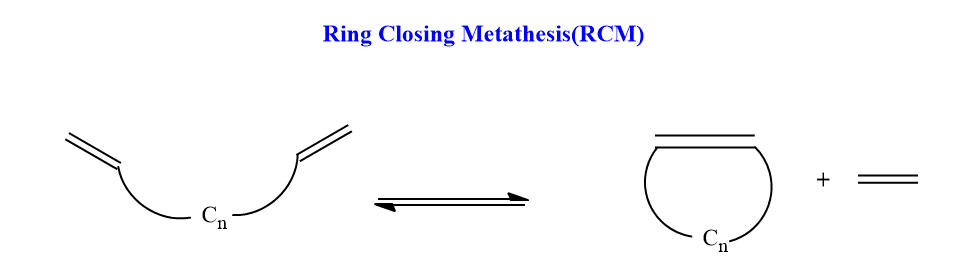
Ring-closing metathesis (RCM)
In this metathesis, the closure of a large ring takes. This is useful in making rings of many different sizes. In this metathesis, long chain diene undergoes ring-closing in presence of a metal catalyst.
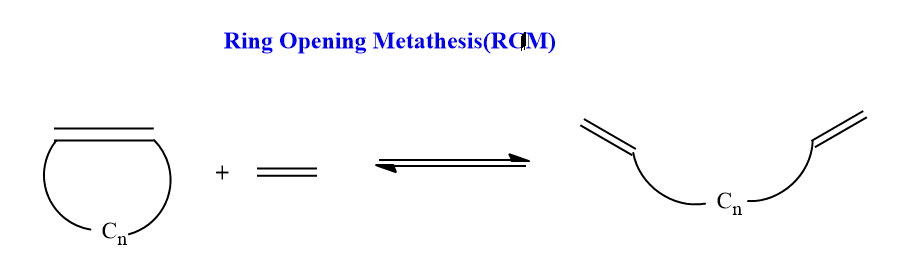
Ring-opening metathesis (ROM)
In this metathesis, the formation of diene from cyclic and acyclic olefins takes place. Cyclic rings that have strained ring often undergoes ROM in presence of another olefin and catalyst.
Ring-opening Metathesis polymerization (ROMP)
In this metathesis, polymerization of cyclic olefins takes place. The reaction uses strained cyclic olefins to produce polymer and copolymer. This ROMP reaction involves a metal carbene catalyst and its mechanism is identical to the normal metathesis mechanism.

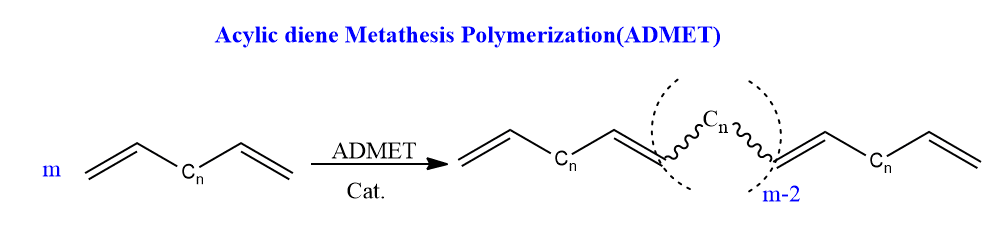
Acyclic diene metathesis polymerization (ADMET)
In this reaction, acyclic diene undergoes polymerization to form long-chain polymers.
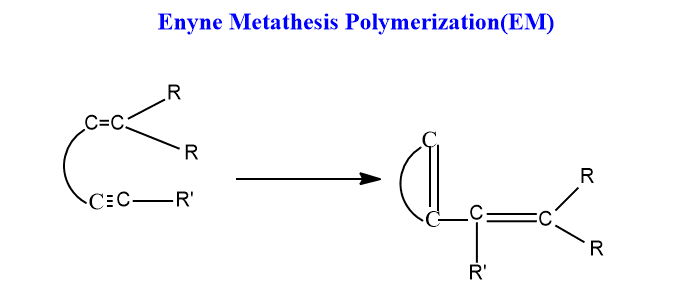
Enyne Metathesis (EM)
Enyne metathesis is defined as the metathesis reaction of an alkyne with an alkene in the presence of a metal carbene as a catalyst, resulting in 1,3-diene products.
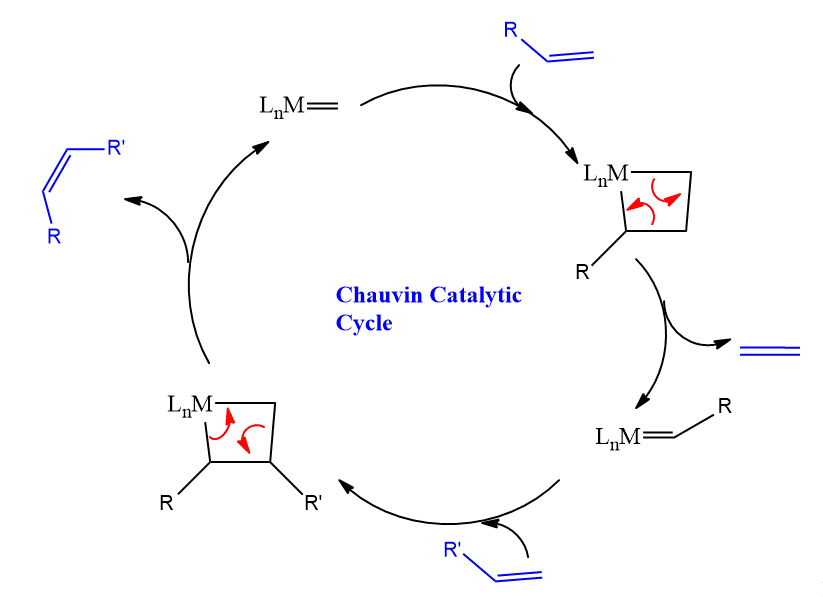
Metathesis reaction mechanism
The Nobel Prize in Chemistry was given to Yves Chauvin, Robert Grubbs, and Richard Schrock in 2005 for their foundational work on the mechanism and applications.
A metal carbene, according to Chauvin and his student Herisson, initiates olefin metathesis. A [2+2] cycloaddition reaction between a transition metal alkylidene complex and the olefin forms an intermediate metallocyclobutane, according to the usually accepted mechanism. The metallocyclobutane then breaks in the opposite direction, yielding a new carbene and an olefin.
As stated above, The metal carbene (methylene) interacts with the alkene to generate the intermediate metallocyclobutane. The intermediate cleaves produce ethylene as well as new metal alkylidenes. One methylene from the catalyst and one from the starting alkene are present in the produced ethene. The metal alkylidene combines with a fresh alkene to produce another metallocyclobutane intermediate, which then cleaves to produce the product and the metal methylene catalyst as shown in the figure.
Importance of Metathesis reactions
Everything from low molecular weight hydrocarbons to sophisticated pharmaceuticals to large molecular weight materials is synthesized via metathesis. Metathesis is used to create particular agricultural chemicals, medicines, and polymers with unique features.
Green advantages of metathesis processes include great atom economy and milder reaction conditions. It is feasible to recycle by-products in metathesis exchange reactions in order to get the maximum possible yields of the desired product.
Metathesis reactions youtube video
What are the examples of metathesis catalysts?
Grubbs catalyst and Schrock’s catalyst are two popular examples of such catalysts.






