Table of Contents
ToggleBenzil benzilic acid rearrangement was first of all reported by Liebig in 1838. Benzil benzilic acid rearrangement is the conversion of benzil (α-diketones) into the salt of α-hydroxyl acid in the presence of an alkali base. This reaction is also known as benzilic acid rearrangement.
Benzilic acid rearrangement
The reaction of 1,2 diketones with alkali hydroxide to produce α-hydroxycarboxylic acids is known as benzilic acid rearrangement.
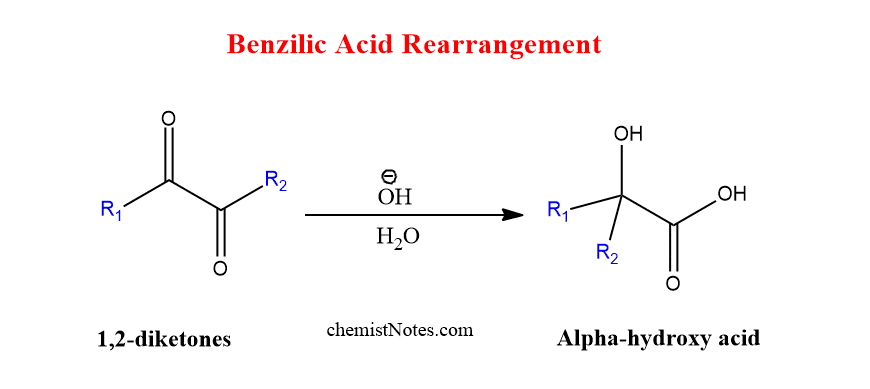
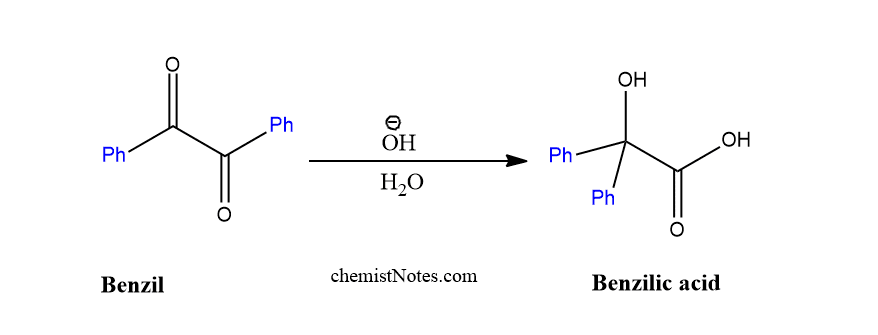
When phenyl group is present in the place of R/R’, then diketone is called benzil, which is rearranged to give benzilic acid in presence of a base.
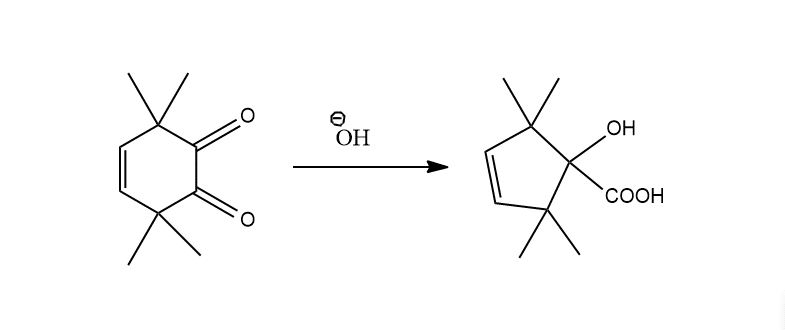
When cyclic diketones undergo this rearrangement reaction, ring contraction takes place.
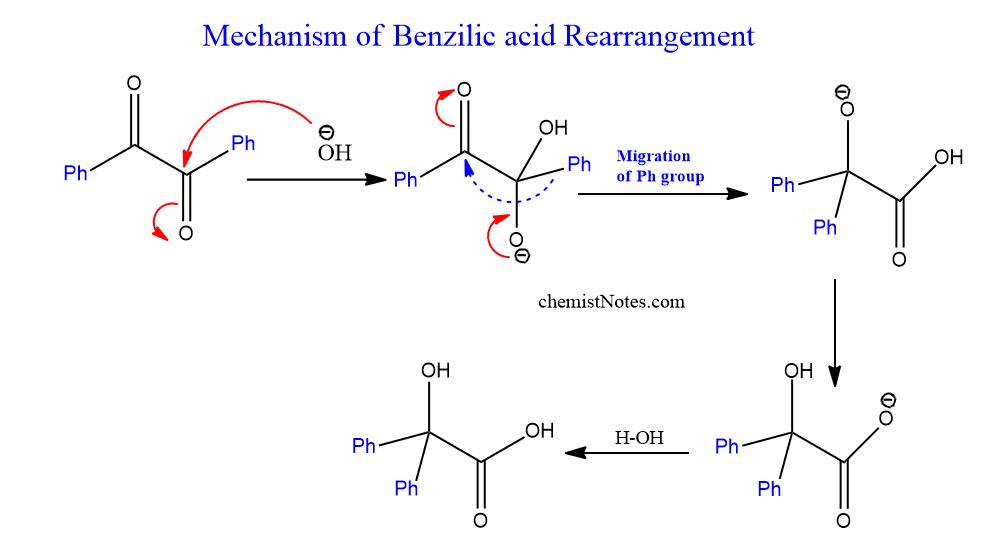
Benzil benzilic acid rearrangement mechanism
Nucleophilic addition of the hydroxide anion to one of the two carbonyl groups initiates the reaction. The corresponding substituents R, attached to the carbonyl group in which hydroxide anion attacks, then move or migrate with bonding electrons to the neighboring carbon atom, resulting in a 1, 2 shift. Finally, a proton transfer caused the carboxylate anion to form. The mechanism is shown below:
Benzilic acid rearrangement applications
This reaction is used to make benzilic acid and its related esters having essential biological properties. The resulting benzilic acid can be utilized to convert alpha, Beta -unsaturated ketones to saturated ketones. Similarly, this reaction can be used in the ring contraction.
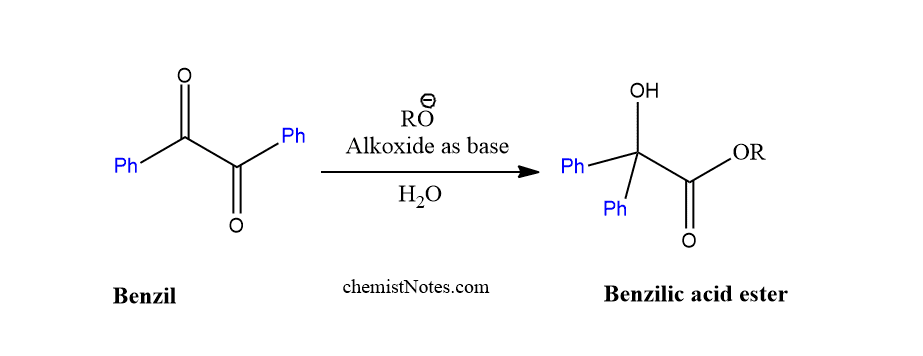
Benzilic ester rearrangement
When alkoxide is used as nucleophile instead of alkali hydroxide, this reaction is called as benzilic ester rearrangement. But the alkoxide should not be sensitive towards oxidation. The reaction product is the corresponding benzilic acid ester.
Benzilic acid rearrangement youtube video
References:
- J.J. Li, Name Reactions, 4th ed.,© Springer-Verlag Berlin Heidelberg 2009
- Wang, Z., Comprehensive Organic Name Reactions and Reagents, John Wiley & Sons, Inc.,2010






