Table of Contents
ToggleHammond postulate is such a hypothesis that helps in determining the shape and geometry of the transition state. Because transition states have no lifetime, they can’t be observed directly, hence information regarding their shape and geometries has to be hypothesized.
Hammond’s Postulate
Hammond’s Postulate states that “For any single reaction step, the geometry of the transition state resembles the side (reactant or product) to which it is closer in free energy“.
For exothermic reactions, the transition state resembles the reactant more than the product.
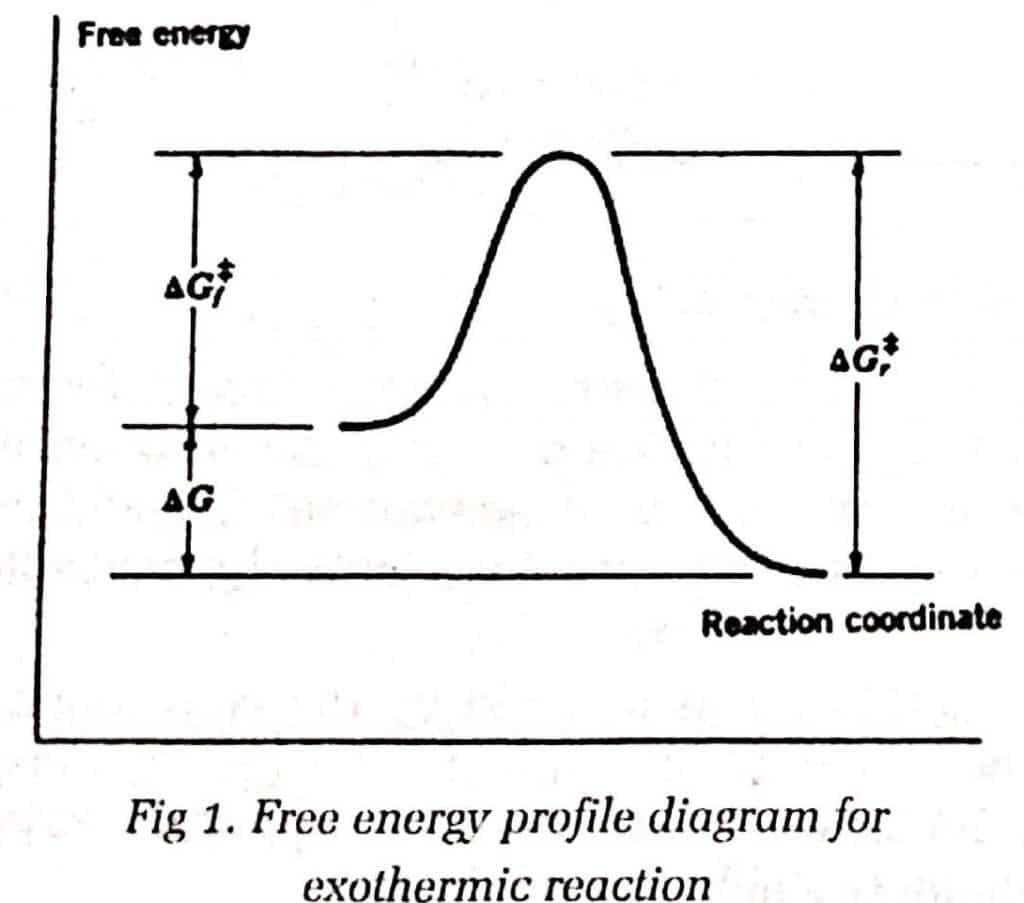
For endothermic reactions, the transition state resembles the reactant more than the product.
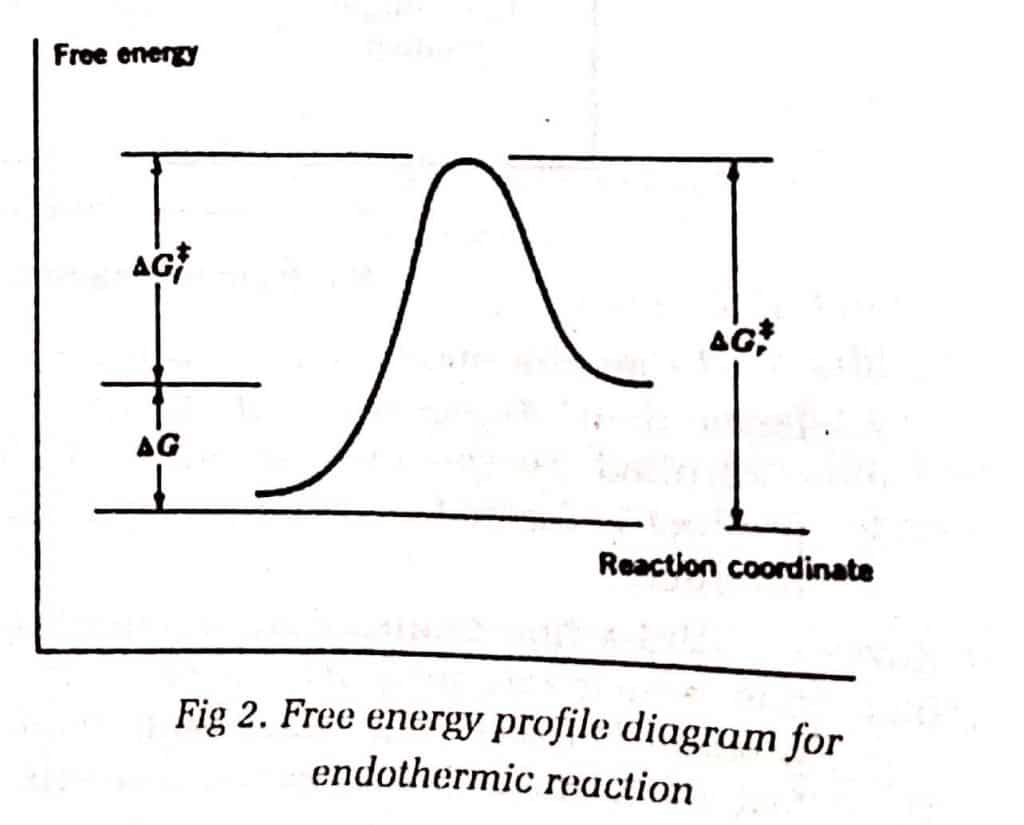
For 2 steps endothermic reaction:
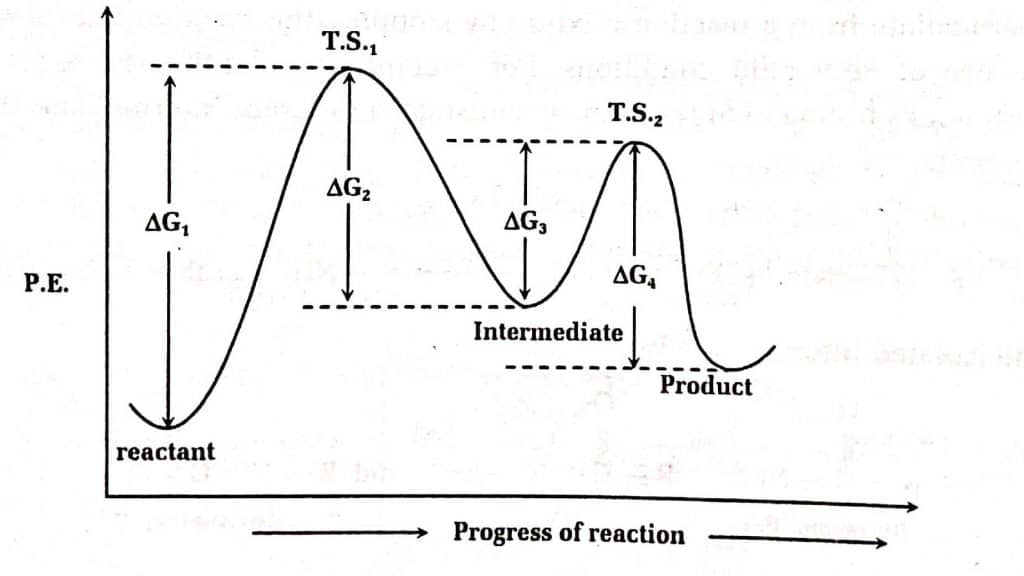
Here, TS1 has lower free energy than the reactants, we may predict that the geometry of the transition state (T.S.) will resemble intermediate than that of the reactants. Similarly, T.S2 has free energy that is closer to that of the intermediate than to that of the products. As a result, both transition phases are more similar to the intermediate than to the products or reactants.
It is very difficult or almost impossible to observe transition states directly since it has zero lifetimes, and hence information regarding their geometry may be obtained from inference. Intermediates, on the other hand, have definite times and can be trapped in a variety of ways, with information about their geometries obtained using a variety of tools and techniques like NMR, mass, Ir spectroscopy, and so on. Thus, we usually prefer intermediate to know the shape and geometry of the Transition state (T.S.)
Application of Hammond’s Postulate
Determining the shape and geometric structure of the transition state in an organic chemical reaction is one of the major applications of Hammond’s postulate..
Hammond’s Postulate Video
References
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988.
- Carey FA, Sundberg RJ (1990). Advanced Organic Chemistry.-Part A: Structure and Mechanism. New York, NY: Plenum.
- Solomons, T.W. Graham & Fryhle, Craig B. (2004). Organic Chemistry (8th ed.). John Wiley & Sons, Inc. ISBN 0-471-41799-8.






