Table of Contents
ToggleMass spectrometry is an analytical spectroscopic technique that involves the ionization of molecules of compounds by bombarding them with an energetic electron beam followed by separating and analyzing the fragments in terms of mass/charge ratio. This technique identifies molecules based on their m/z ratio. It has wide applications in qualitative and quantitative analysis. The general introduction of mass spectrometry, its basic principle, ionization methods, analyzers, instrumentation, and applications have been discussed in this blog.
Mass spectrometry principle(MS)
The basic principle of mass spectroscopy or mass spectrometry is based on the fact that only the charged particles generated from the compounds during the ionization process are detected and the corresponding mass spectrum is obtained. There are different techniques of ionization(mentioned below) but for simplicity, we have explained only the electron impact ionization method.
In this technique, a given compound is vaporized first and then the molecules of the compound are bombarded with an energetic electron beam. Due to collision, the energy of electrons is transferred to molecules which in turn emit electrons to produce positively charged ions called molecular ions or parent ions, or biradical cations. These molecular ions are highly energized and often undergo fragmentation to give many smaller positive ions called daughter ions. Each of the daughter ions has a certain value of m/z charge. Mostly the ions produced in fragmentation carry a +1 charge hence, m/z usually represents the mass of the ion.
Thus, obtained molecular and daughter ions are passed through an electric field and strong magnetic field. Therefore, molecular and daughter ions having an m/z ratio get separated and collected at the detector.
A signal is obtained corresponding to each m/z value and the intensity of the signal is proportional to the number of ions giving rise to the signal.
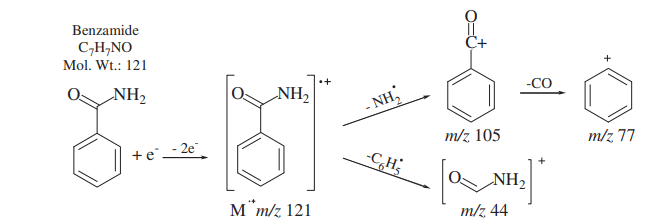
Mass spectrometry fragmentation
The fragmentation of Benzamide in mass spectroscopy is shown:
Mass spectrometry graph
A mass spectrum is a graph between relative abundance or intensity versus the m/z value of ions. The mass spectrum(graph) of Benzamide is shown below:
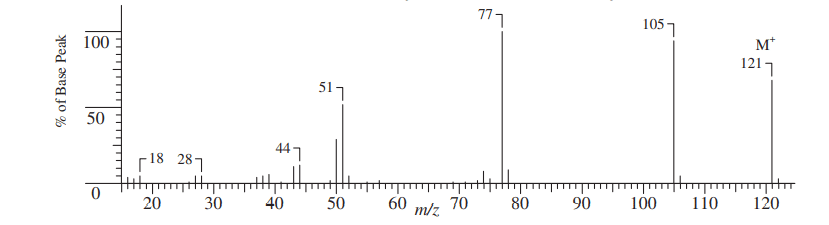
Base peak in mass spectrometry
The ions formed by removing one electron from the parent molecules are known as molecular ions or parent ions and the corresponding largest peak found in the mass spectrum is called the base peak. The molecular ion peak is usually denoted by M+. It may or may not be the peak of the highest intensity. The molecular ion is the most important ion as its mass is the molecular mass of the parent molecule.
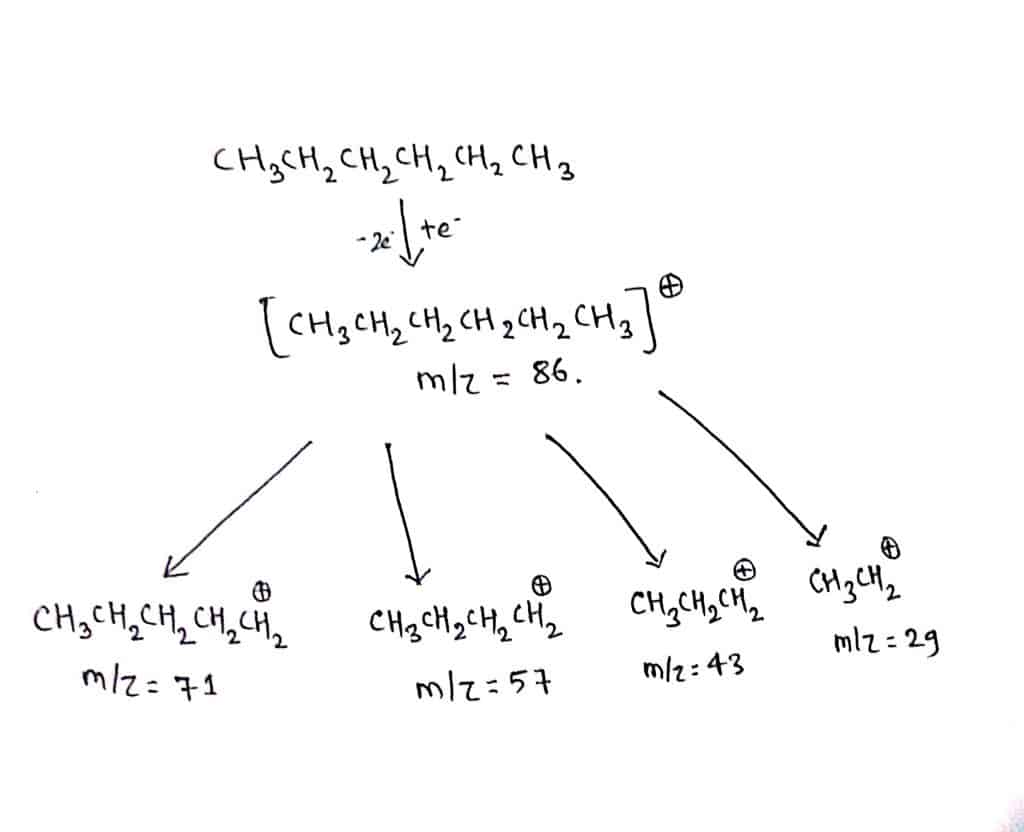
Fragmentation of hexane
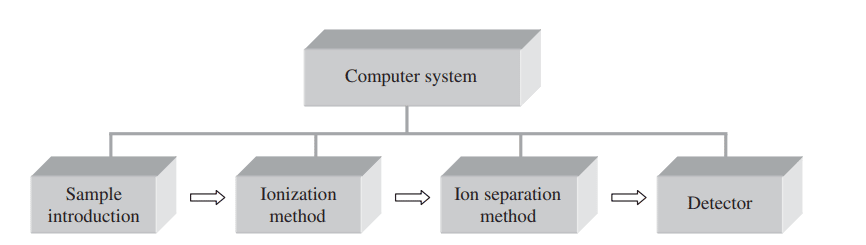
Mass spectrometry instrumentation
The block diagram of the typical mass spectrometer is shown below:
Different types of ionization techniques in mass spectrometry
In a mass spectrometer, a compound is first vaporized and converted into ions, which are then separated and detected. The most common ionization technique involved in mass spectroscopy are listed below:
- Electron impact ionization
- Chemical ionization
- Field desorption ionization
- Fast atom bombardment ionization
- Plasma desorption ionization
- Laser desorption ionization
- Thermospray mass spectrometry
- Electrospray mass spectrometry
Types of analyzers
The mass analyzer is the heart of each mass spectrometer which separates the mixture of ions that are generated during the ionization step by m∕z in order to obtain a spectrum. There are several different types of analyzers with different characteristics
- Magnetic sector mass spectrometer
- Quadrupole mass spectrometer
- Ion trap mass spectrometer
- Time of flight mass spectrometer
- Fourier transform mass spectrometer
Application of mass spectroscopy
Mass spectrometry has both qualitative and quantitative uses.
A. Qualitative analysis
- Identification of an unknown compound by comparing its spectra with a known sample
- Determination of molecular structure by analyzing fragmentation patterns, and their relative intensities in the mass spectrum.
- Characterization and determination of sequencing of amino acids in proteins.
- Determination of the isotopic composition of elements within a sample.
B. Quantitative analysis
- Determination of molecular mass and molecular formula by observing mass spectrum and fragmentation pattern.
- It is widely used to determine the size of the nanoparticles.
- It is also applicable in biotechnology, pharmaceutical, clinical, and environment.
Mass spectrometry video
FAQs/MCQs:
What is mass spectrometry?
Mass spectrometry is an analytical spectroscopic technique that involves the ionization of molecules of compounds by bombarding them with an energetic electron beam followed by separating and analyzing the fragments in terms of mass/charge ratio.
What is the base peak in mass spectrometry?
The ions formed by removing one electron from the parent molecules are known as molecular ions or parent ions and the corresponding largest peak found in the mass spectrum is called the base peak.
What does mass spectrometry tell?
Mass spectrometry tells about the molecular mass and molecular structure of a compound.
What is the molecular ion in mass spectrometry?
The ions formed by removing one electron from the parent molecules are known as molecular ions.






