Table of Contents
ToggleElectrophilic allylic rearrangement is quite similar or analogous to nucleophilic allylic rearrangements. This is actually an electrophilic substitution reaction taking place at the allylic substrate.
Electrophilic substitution accompanied by double bond shifts
When electrophilic substitution is carried out at an allylic substrate, the rearranged product may be formed, hence this reaction is called electrophilic allylic rearrangement reaction.
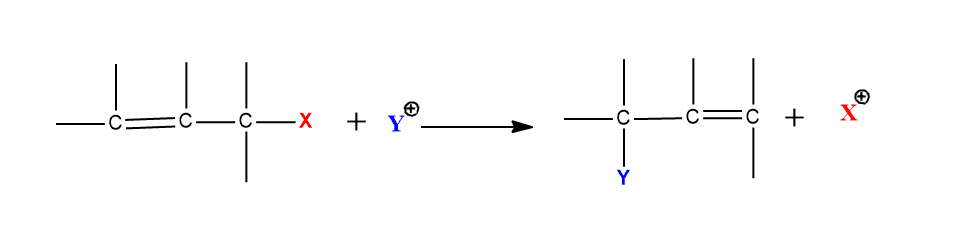
There are two principal mechanisms to explain this process.
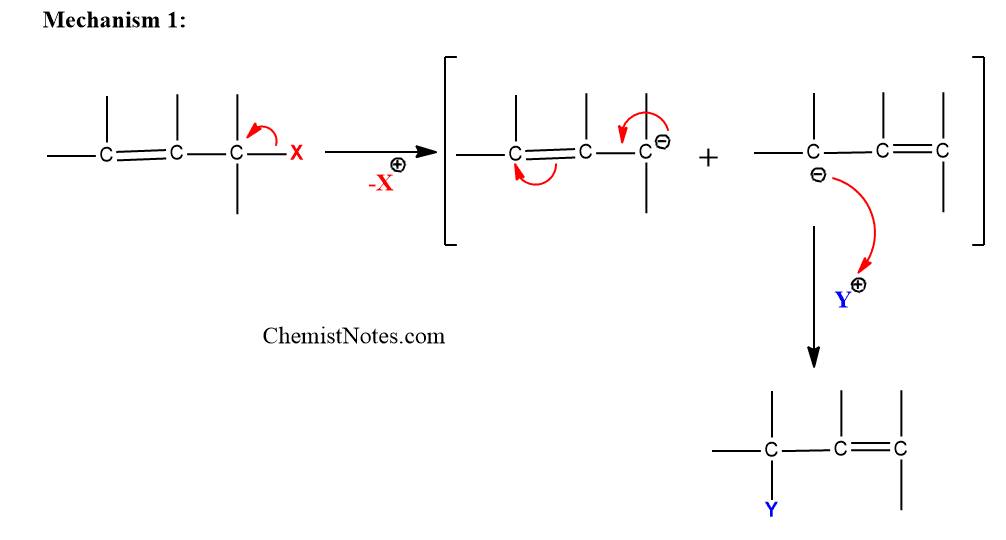
Mechanism 1: In this mechanism, the leaving group is removed initially to give a resonance stabilized allylic carbanion. Then electrophile attack on the resonance stabilized the carbanion.
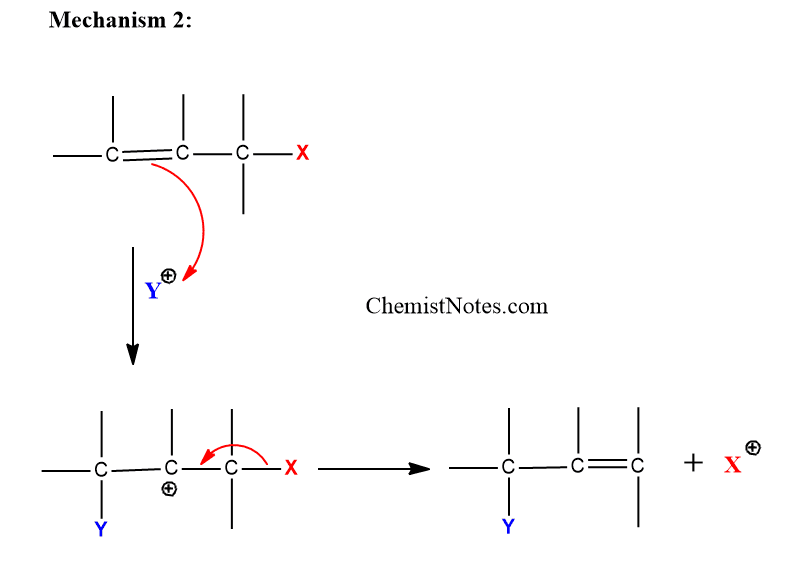
Mechanism 2: In this mechanism, the electrophile Y attack the substrate at first giving carbanion. Then, leaving group X gets detached.
Generally, hydrogen is leaving the group in such a type of electrophilic allylic rearrangement. Although the geometry of electrophilic allylic arrangement has not been well investigated, most rearrangements occur with anti stereoselectivity.
Electrophilic Allylic Rearrangement Example
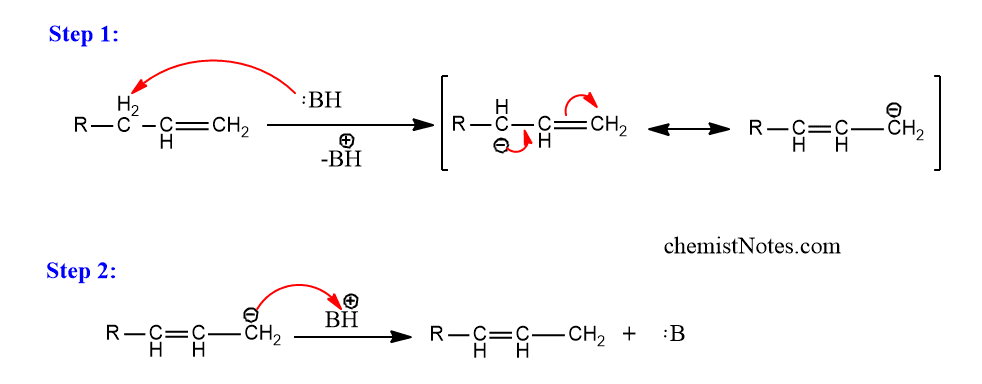
The double bonds of many unsaturated compounds change when they are treated with strong bases. In many circumstances, equilibrium mixtures are formed, with the most thermodynamically stable isomer dominating. Thus, if a new double can be in conjugation with one already present or with an aromatic ring, the double bond goes that way.
This type of reaction is referred to as prototropic rearrangement, and it involves an electrophilic substitution with allylic rearrangement. The mechanism involves the abstraction of a proton by the base to produce a resonance stabilized carbanion, which is then combined with a proton at the site that produces the more stable olefin.
References
- J. March, Advanced Organic Chemistry, (4th Edition), John Wiley and Sons, 1992.






