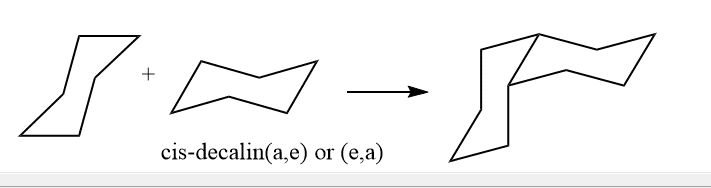
Table of Contents
ToggleDecalin is formed by the fusion of two 6-membered rings and is the most important fused ring among others. The IUPAC name for decalin is bicyclo[4.4.0] decane. Similar to cyclohexane, the six-membered rings of decalin are expected to be most stable in the chair form. However, joining two chairs is a possibility in two different methods. Decalin can exist in cis-decalin and trans-decalin forms among them trans one is more stable.
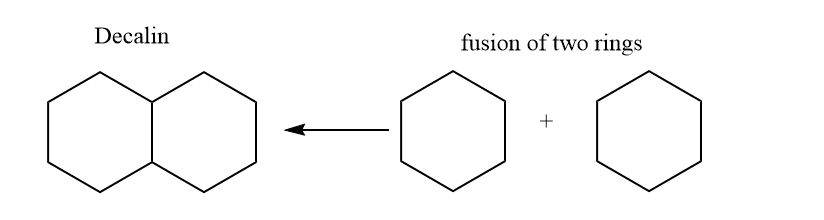
Decalin structure
Decalin is the most important among the fused 6-membered rings with 3,4 and 5-membered rings. The name of decalin is bicyclo[4.4.0] decane without bridgehead carbons. Fused rings can be formed in two forms that are cis and trans. Cis fusion takes place through two nearly eclipsed bonds whereas trans fusion by deformation. There are different fused rings known they are hydrindane that is bicyclo[4.3.0] nonane, decalin, perhydrophenanthrene, perhydroanthrancene, etc In this article we are going to study stereochemistry, conformation, and stability of Decalin.
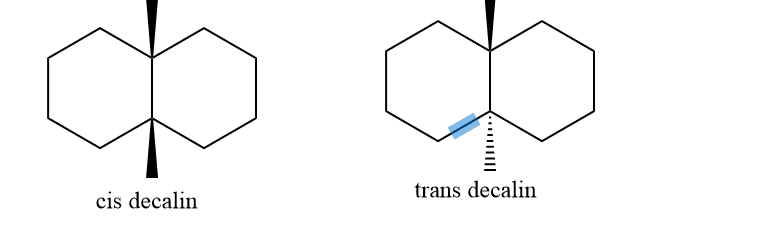
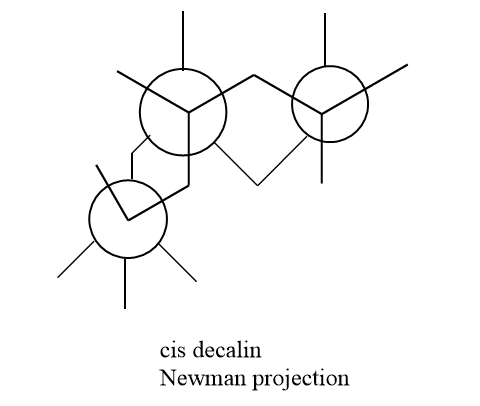
In the cis form, the two rings are cis-fused. Similarly, in Trans form, the two 6-membered rings are trans-fused. In cis same as the conformation of disubstituted cyclohexane, one substituent will be axial and another will be Equatorial and in trans, both will be either axial or equatorial. Cis isomer of decalin has two interconvertible chair forms which are enantiomer.
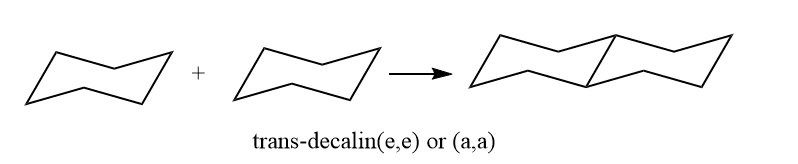
By use of equatorial and equatorial bonds, the trans decalin is fused. Because the fusing of two cyclohexane rings through two axial bonds results in a highly stained molecule, it has a rigid structure and cannot undergo ring inversion, whereas a,e bond interchange at ring junction is simple in the cis form.
Decalin stereochemistry
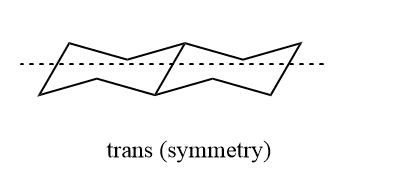
The simple rotation of groups around carbon-carbon bonds is unable to bring about the interconversion of cis and trans decalin. Decalins resemble 1,2 disubstituted cyclohexane on this basis. Each cyclohexane ring in trans decalin is linked by an equatorial bond, as is mentioned. In trans decalin, it causes gauche interaction to cancel inside a single ring, just like in cyclohexane. Because there is no gauche methylene interaction, trans decalin does not contain any gauche units. But in cis decalin, there are at least three gauche interactions which can be shown below.
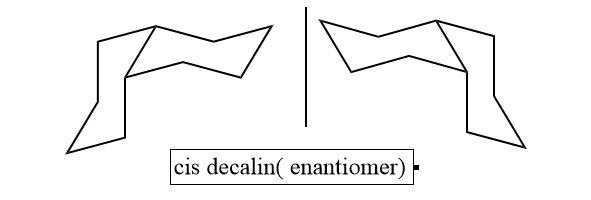
Cis and trans decalin can be distinguished by NMR spectra as cis shows only one proton peak whereas trans shows two protons peaks. cis-Decalin is a chiral molecule without a chiral center; it lacks reflective symmetry but has a two-fold rotational symmetry axis. A chair-flipping process, however, cancels the chirality by making the molecule into its mirror image.
Symmetry in decalin
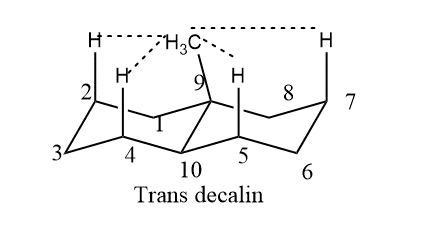
As we know, if there is any symmetry present in compounds, they became achiral and optically inactive. Trans decalin shows a center of symmetry and is optically inactive. And has a C2h point group. Cis decalin has two-fold axis symmetry passing through the midpoint of the C9 and C10 bond dissecting the dihedral angle between 9-H and 10-H. It has therefore total symmetry number 2 belongs to point group C2.
Substituted decalin
Among the decalin substitutes. The 9 methyl decalin system is the most significant because it is present in steroids and many other natural compounds. In trans decalin, the substituents in an angular position are axial with respect to both rings, whereas, in cis decalin, they are equatorial with respect to some rings and only axial with respect to one ring. When compared to the cis isomer, the methyl groups in trans decalin interact with four synaxis hydrogen atoms on C2 and C4, respectively.
Decalin Newman projection
Decalin uses
The substituted decalin which is 9 methyl decalin is generally used in steroids, and many natural products. The industrial solvent decalin is used to dissolve naphthalene, fats, resins, oils, and waxes. Along with replacing turpentine in lacquers, paints, and varnishes, it serves as a solvent and stabilizer for floor and shoe polishes as well as a component of motor fuels and lubricants. Used as paint thinner and paint remover.
Decalin fuel additive
Two types of additives are present in Decalin RunUp: one is intended to reduce the negative effects of tetraethyl lead in aviation fuel, and the other is intended to increase combustion efficiency, reduce combustion deposits, and clean the fuel delivery system in aircraft.
Decalin price
| Size | Price |
| 500mL | 1900 INR |
| 2500mL | 9000INR |
| 500mL | 3040 Rs |
| 500mL | $36 |
FAQs/MCQs
What is the IUPAC name of decalin?
The IUPAC name of decalin is bicyclo [4.4.0] decane.
Is decalin optically active?
Trans-decalin is optically inactive and achiral because it has an optically active plane of symmetry and an optically active center of symmetry.
Which form of decalin is more stable?
Decalin’s six-membered rings are predicted to be most stable in the chair form, similar to cyclohexane’s.
Why the cis form of decalin is optically active?
The cis form has non-superimposable mirror images and no centers of symmetry in either of its two forms, whereas the trans form has a center of symmetry and is optically active.






