Table of Contents
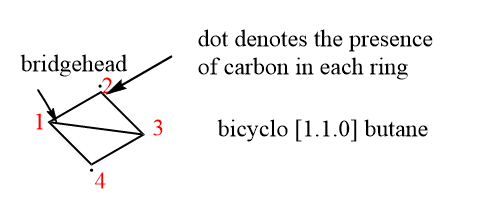
ToggleA fused ring is a compound of two or more rings in which the adjacent rings share at least two atoms. In fused rings, bridgehead carbons are directly connected. The bicyclo [1.1.0] butane is the fused ring that is the smallest and most strained. Some examples of fused rings are :
- Rolipram.
- Telomestatin.
- Phosphotransferase.
- Malonaldehyde.
- Proton.
- Ligand.
- Pyrazole Derivative.
- Alkadiene.
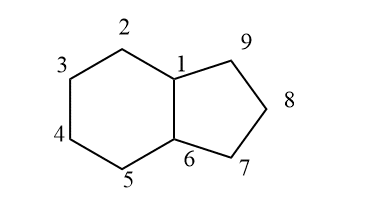
- decalin
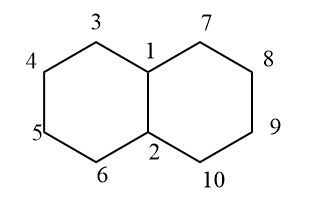
- hindrindane
Fused rings definition
Fused rings are those in which two nearby carbon atoms are shared without bridgeheads carbons. Spirans, on the other hand, are compounds in which two rings share a single carbon atom. Bridged rings are produced when two or more atoms share space in two rings.
Nomenclature of fused rings
The naming for the fused rings is simple to understand. First, we have to understand that we have to name it bicyclo and bicyclo is kept at the starting then, we have to count the number of carbon on both sides between the bridgehead and the number of carbon is written in the bracket. Numbering started from the largest ring to the smallest one then we have to write the name of the parent chain. We can easily understand the nomenclature by the following examples for a clear vision.
![bicyclo [2.1.0] pentane](https://chemistnotes.com/wp-content/uploads/2023/01/b8.png)
Let us understand this above example, in the fused ring, we have 2 carbon in the largest ring and 1 in the smallest that’s we have to write 2.1 and there is no carbon in the bridgehead so we have 0 in that bracket and the parent chain is pentane that’s why the name is written bicyclo [2.1.0] pentane.
![bicyclo [2.2.0] hexane](https://chemistnotes.com/wp-content/uploads/2023/01/b9.png)
![bicyclo [3.2.0] heptane](https://chemistnotes.com/wp-content/uploads/2023/01/b11.png)
Preparation of fused rings compounds
The highly strained bicyclo [1.1.0] butane can be generated by 1,3-dihalocyclobutane with metallic sodium under suitable conditions. There are various methods to prepare such fused rings.
Fused rings can be formed in two forms that are cis and trans. Cis fusion takes place through two nearly eclipsed bonds whereas trans fusion by deformation. There are different fused rings known they are hydrindane which is bicyclo[4.3.0] nonane, decalin, perhydrophenanthrene, perhydroanthrancene, etc The following methods are used to prepare fused ring compounds.
- By the reaction of 1,3-dihalocyclobutane with metallic sodium under suitable conditions.
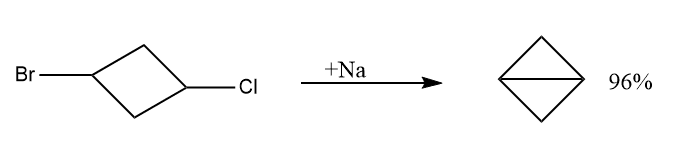
2. By the internal addition reaction of allyl carbene
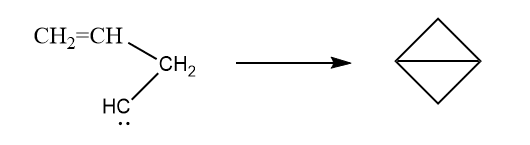
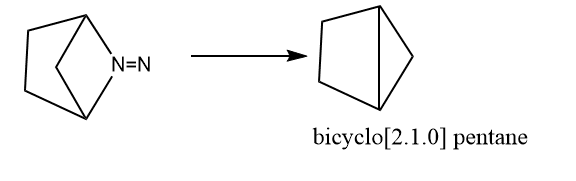
3. By the pyrolysis of bicyclic azo compounds
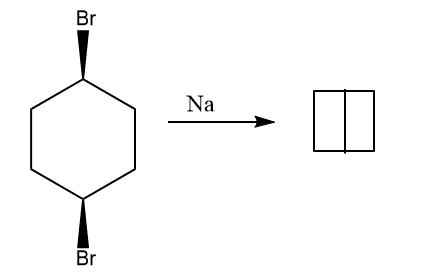
4. By treating cis-1,4-dibromocyclohexane with sodium
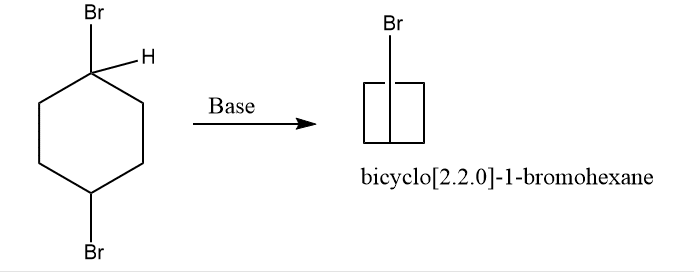
5. When 1,4- dibromocyclohexane is treated with base
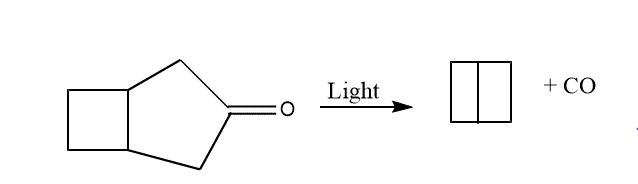
6. By photolysis
Fused rings generally exist in cis and trans forms. And cis form is more stable than the trans one.






