Table of Contents
ToggleGas Chromatography is a separation technique used in analytical chemistry for the separation of a mixture of compounds that are volatile. It provides both the broad analysis of the total sample and the specific information on individual components of the sample for a simple to the complex mixture of compounds. In gas chromatography, the mobile phase is a chemically inert gas, while the stationary phase is either solid (in gas-solid chromatography), or liquid (in liquid-gas chromatography). Gas Chromatographic technique was developed by Martin and Synge
Gas Chromatography Principle
The sample solution injected into the instrument is distributed between the two phases; stationary phase and mobile phase. The sample components are carried by a gas stream (mobile phase) through the stationary phase into a separation tube known as the “column”, where the various components are separated.
Because each component’s properties and structures differ, the size and affinity of each interaction with the stationary phase vary. As a result, even when the driving force is the same, the retention duration of individual components in the column varies, causing them to exit the column in different orders. Thus, the components of the sample are separated based on the principle of partition chromatography.
How does gas chromatography work?
If the sample to be injected into carrier gas is liquid, it must be evaporated well. Then, the sample is passed into the mobile phase, a stream of Hydrogen (carrier gas) that passes over the packed column. The components are separated inside the column by the differential partition between the mobile phase gas and the stationary phase. The degree of interaction between each component and the stationary non-volatile phase affects the rate. Substances that interact more with the stationary phase are retarded, and hence separated from those that do not. Then, the components eluted from the column are detected by the detector for further analysis.
Gas Chromatography instrumentation
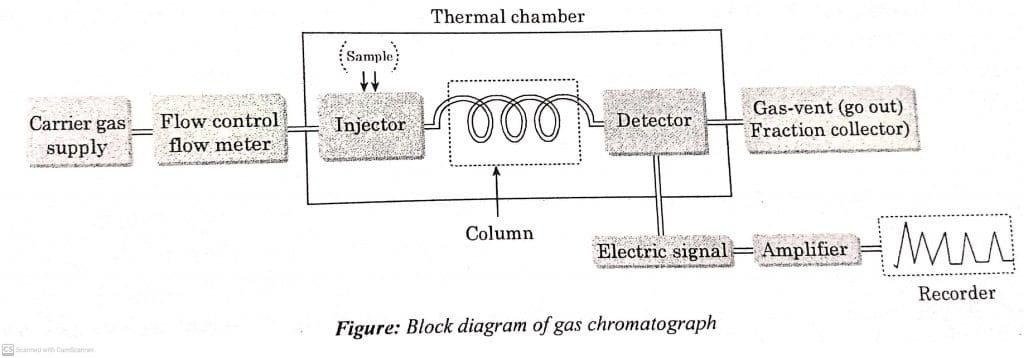
Gas Chromatography consists of the following parts:
- Carrier gas supplier: It is a mobile phase consisting of inert gases such as Hydrogen, Helium, Argon etc. The choice of carrier gas depends on nature of the sample, consumption, column efficiency, type of detector and so on.
- Sample injector: The sample injected usually ranges from 0.1-50 micro litre for gases and little amount of mg for solids. The sample that is carried out by the gases (mobile phase) to the column is coonected to injector. It is injected through syringe and heated to rapid vaporization.
- Columns: The columns are stainless steel, copper, nickel or plastic tubes. There are two types of column in gas chromatography i.e. packed columns and capillary column. Packed column is generally used when the sample is large. Capillary column offers greater resolution in comparison with packed column.
- Detector: The detector is a transducer in which the input chemical signal is converted into output electric signal. It is used for the continious measurement of solute concentration of the carrier gas stream. The ideal detector should have the characteristics like high sensitivity, rapid response, and high signal to noise ratio.
- Recorder: The signal from detector passes through amplifier, and then to the recorder. The recorder consists of mobile recording pen, and a recording chart strip.
Application of Gas Chromatography
- Used for the separation of volatile mixtures
- Used for the qualitative and quantitative analysis of food
- Detection and analysis of contaminants such as environmental pollutants, pesticides, and naturally occurring toxins
- Used for the quality control of chemicals in medicines, automobiles, and so on
- Used for the analysis of natural products and to detect blood alcohol levels.
- Separation of hundred of hydrocarbons petroleum by GLC
- Also used to study reaction mechanism
Advantages of Gas Chromatography
- Fast analysis time
- High detection sensitivity
- Good separation efficiency
- Requirement of small sample for the analysis
Gas Chromatography-Mass Spectrometery (GC-MS)
GC-MS is the combination of Gas Chromatography (GC) and Mass Spectrometry (MS), used in analytical chemistry to analyze different constituents present in the sample mixtures. In gas chromatography, the sample is carried by inert gases (mobile phase) over the column containing the stationary phase.
The components are separated inside the column by the differential partition between the mobile phase and the stationary phase. As each of the components has a different affinity for the stationary phase, they get eluted at different times, also called retention time, and hence these eluted components get captured by a mass spectrometer, which is then ionized (mass to charge ratio), subjected to electromagnetic field and to the detector. The combination of these two techniques provides an accurate constituent of interest in the sample. It has been found to be widely used in forensic laboratories, quantification of organic contaminants, anti-doping tests, and so on.
FAQs
What is gas chromatography?
Gas Chromatography is a separation technique used in analytical chemistry for the separation of a mixture of compounds that are volatile.
gas liquid chromatography
If the stationary phase in gas chromatography is liquid, then it’s called gas chromatography. In gas-liquid chromatography, the mobile phase is chemically inert gases, while the stationary phase is liquid.
gas chromatography retention time
Each of the components has a different affinity for the stationary phase when the mobile phase containing sample components is forced to pass through the column (stationary phase), they get eluted at different time. This time is the retention time.
gas chromatography stationary phase
In gas chromatography, the mobile phase is a chemically inert gas, while the stationary phase is either solid (in gas-solid chromatography), or liquid (in liquid-gas chromatography).
gas chromatography definition
Gas Chromatography is a separation technique used in analytical chemistry for the separation of a mixture of compounds that are volatile.
Mobile phase in gas chromatography
The mobile phase in gas chromatography is carrier gases / chemically inert gases.
What is gas chromatography used for?
Gas chromatography is used for the separation of volatile mixtures, detection, and analysis of contaminants such as environmental pollutants, pesticides, and naturally occurring toxins, and so on.
What is the effect of temperature on gas chromatography?
The temperature has a very significant effect on gas chromatography in terms of retention, peak shape, and selectivity. For every 15 degrees celsius greater or lower temperature, the retention of column increases or decreases by a factor of 2.
What are the types of Gas Chromatography?
Based on the stationary phase, gas chromatography is of two types: gas-liquid chromatography (stationary phase is liquid), and gas-solid chromatography (stationary phase is solid).
gas chromatography ethanol analysis
Ethanol and water analysis in gas chromatography is done by using Ionic Liquid Capillary Gas Chromatography with Thermal Conductivity Detection technique.
What are the types of Detectors?
There are 4 types of detectors: Flame-Ionization Detector (FID), Electron-Capture Detector (ECD), Thermal Conductivity Detector (TCD), and Nitrogen-Phosphorus Detector (NPD)






