Table of Contents
ToggleDefinition: Column Chromatography is one of a purification and separation technique in which a mixture of components are purified and separated with the assistance of column is called column chromatography. The column is a tubular structure made up of glass or plastic.
In column chromatography, the stationary phase is solid (Eg: silica, alumina, cellulose, ion exchange resins, beads, etc), whereas the mobile phase is liquid. In column chromatography, the entire purification and separation process occurs in a tube-like column.
Principle of column chromatography
Components of the mixture have different rates of movement along the column. Components having lower affinity and adsorption to stationary phase move faster as compared to components having higher adsorption and affinity with the stationary phase. So, the components that travel fast are removed first from the column, whereas the components that travel slowly are eluted at last.
Types of Column Chromatography
On the basis of the stationary phase used, column chromatography can be categorized into the following groups:
- Adsorption chromatography: here the stationary phase is adsorbent material like alumina, silica. If the silica is used as stationary phase it is called silica gel column chromatography.
- Ion exchange chromatography: in this type of chromatography ion exchange resins are used as stationary phase.
- Affinity chromatography: here the stationary phase is linked with ligand which binds specific molecules (like enzyme, proteins)
- Gel permeation chromatography: in this type stationary phase is porous gel or beads.
Instrumentation/ set up of column chromatography
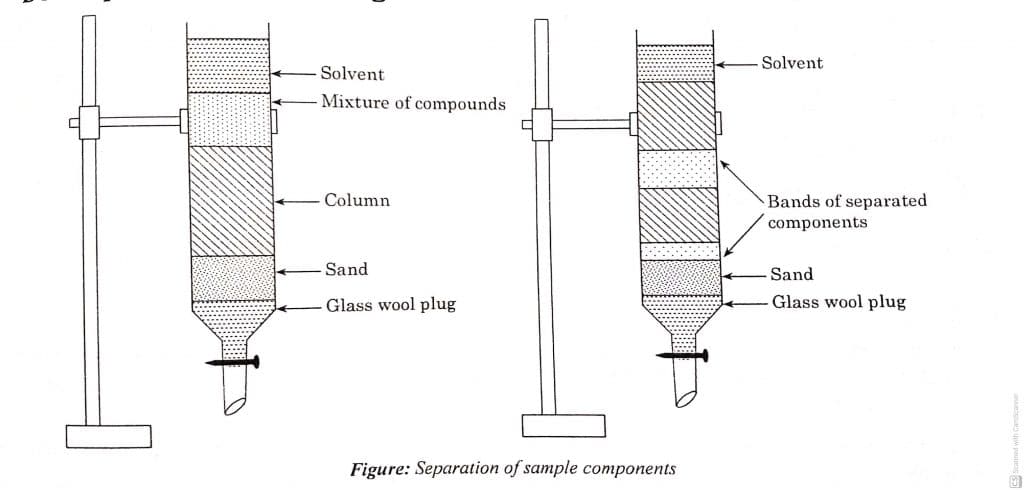
Overall, the instrumentation of column chromatography consists of the following components.
- Column: tubular structure in which separation and purification occurs.
- Stationary phase: solid acts as stationary phase.
- Mobile phase: liquid acts as stationary phase.
How does column chromatography works?
Column chromatography works in the following sequential steps:
- Selection of stationary phase
The stationary phase is selected according to the nature of the sample depending on how the sample is purified (size, polarity, charge). Usually, silica or alumina are used as stationary phases.
2. Packing of column
Packing of column means inserting stationary phase into the column. Since the column is open from both sides, it should be closed by using the proper amount of cotton plugs at the bottom sides. Column packing can be done in two ways
- Dry method/packing
At first, the stationary phase (eg: powdered silica) is filled first and then only the solvent solution that acts as the mobile phase is run.
- Wet method/packing
In this procedure, stationary phase and solvent mixed togetherly that lead to the formation of slurry and then this formed slurry is packed in a column. Wet packing is more preferable to dry packing because of the lower requirement of solvent solution in wet packing than in dry packing.
During packing air bubbles should be completely avoided.
3. Selection of mobile phase
Single solvent or mixture of solvent can be used as mobile phase. Mobile phase is chosen according to stationary phase. If stationary phase is polar then mobile phase should be one that is less polar or non-polar or vice versa.
The sample to be purified is run along with mobile phase is column. More polar compounds of the sample move with higher speed because polar compounds show greater affinity with the polar stationary phase.
Elution can be done in two ways in column chromatography
- Isocratic elution
In this technique, the same concentration of a single solvent is employed in the entire process.
- Gradient elution
Here, different concentrations of the same solvent or different solvents are used (if the sample is complex). Initially, the less polar solvent is used then the slightly more polar solvent is used for elution. Finally, different elution is taken for analysis.
Applications of Column Chromatography
- Applicable for isolation of metabolites from biofluids.
- Used to remove impurities from sample, hence to purify the sample.
- Applicable for the separation of individuals components from the mixture.
- A chemist uses it figure out how much drug is in a drug sample.
- It is utilized to separate diastereomers, isolate racemates and geometrically separate isomers.
Advantages
- Impurities are separated from any mixture.
- Separation of any kind and amount is conceivable.
- The procedure is low cost and straightforward to comprehend.
- A wide range of solvents can be utilized in the procedure to get desired output.
Limitations
- It is laborious and time consuming process.
- Since, it is a long procedure one will need to pay attention and focus the entire time.
- In the separation process, little quantities become insufficient only a large may be employed for separation.
FAQs
Silica gel column chromatography
In Adsorption chromatography, here the stationary phase is adsorbent material like alumina, silica. If the silica is used as stationary phase it is called silica gel column chromatography
What is column chromatography?
Column Chromatography is one of a purification and separation technique in which a mixture of components are purified and separated with the assistance of column is called column chromatography.
Affinity column chromatography
Here the stationary phase is linked with a ligand that binds specific molecules (like enzymes, proteins)
Column Chromatography Packing
Packing is done in two types: wet packing and dry packing






