Table of Contents
ToggleChromatographic rate theory: The rate theory of chromatography describes the shapes and breadths of column bands in quantitative terms based on a random-walk mechanism for the migration of molecules through a column. Due to the several limitations of the plate theory, rate theory has been widely used. It describes the effect of an elution band as well as its time of elution. It is based upon the Van-Deemter equation that describes the relation of a theoretical plate H and the average linear velocity of the mobile phase.
H = A + Bu + c u
Where H = Height equivalent to theoretical plates
A = Eddy’s diffusion
c = mass transfer
u = flow rate of mobile phase/average mobile phase velocity
The rate theory which describes the properties of chromatographic separation by comparing the rate of analyte that elutes through column provides a more realistic description of the process that works inside a column.
Eddy’s diffusion
In Eddy’s diffusion, the mobile phase moves through the column i.e. stationary phase packed with porous silica. As the packing of the column is uneven and has interparticle spaces, the path of the analyte will not be the same for all the molecules of the analyte i.e. they do not all reach the detector at the same times, and hence result in band broadening.
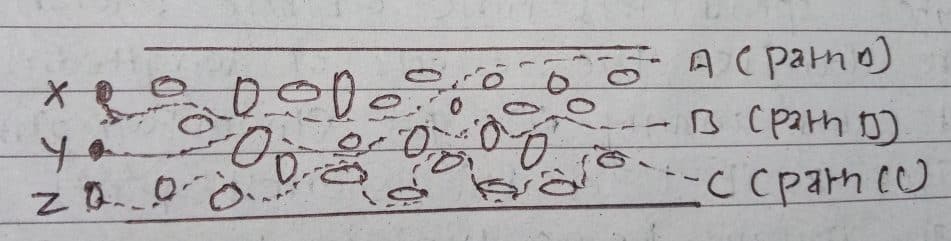
If the analyte has three molecules i.e. X, Y, and Z, then particle ‘X’ takes path A which is the shortest path. Path B is taken by particle Y that is a comparatively larger path than path A, while particle Z takes the longest path C. The particle that has chosen the shortest path elutes first and therefore the path length order becomes A < B < C. The longer the path length of the molecule, the more be elution time and hence band broadening occurs. This effect is called Eddy’s diffusion.
Eddy’s diffusion occurs in HPLC as the packed column is used in this technique. The longitudinal diffusion is zero/negligible.
Longitudinal diffusion
As the concentration of the analyte is less at the edge of the band in comparison to the center, the analyte diffuses from the center to the edges. In other words, when the sample passes through the column, it takes multiple paths and moves/diffuses from the high concentration center path to low concentration extreme sides. This results in band broadening. If the velocity of the mobile phase is high, then the analyte spends less time on the column and hence decreases the effect of longitudinal diffusion. It occurs mainly in the gas chromatographic technique as it takes the shortest path and capillary columns are used in the gas chromatography. Hence, the eddy’s diffusion is negligible or zero.
Mass transfer
Mass transfer is simply the movement of the particles. Larger particles diffuse slowly while the shorter particles move fast and elute fast too. If the velocity of the mobile phase is high, then the affinity of the analyte for the stationary phase becomes strong, and hence the analyte in the mobile phase will move ahead of the analyte in the stationary phase. This results in band broadening of analytes. The higher the velocity of the mobile phase, the worse the broadening becomes.
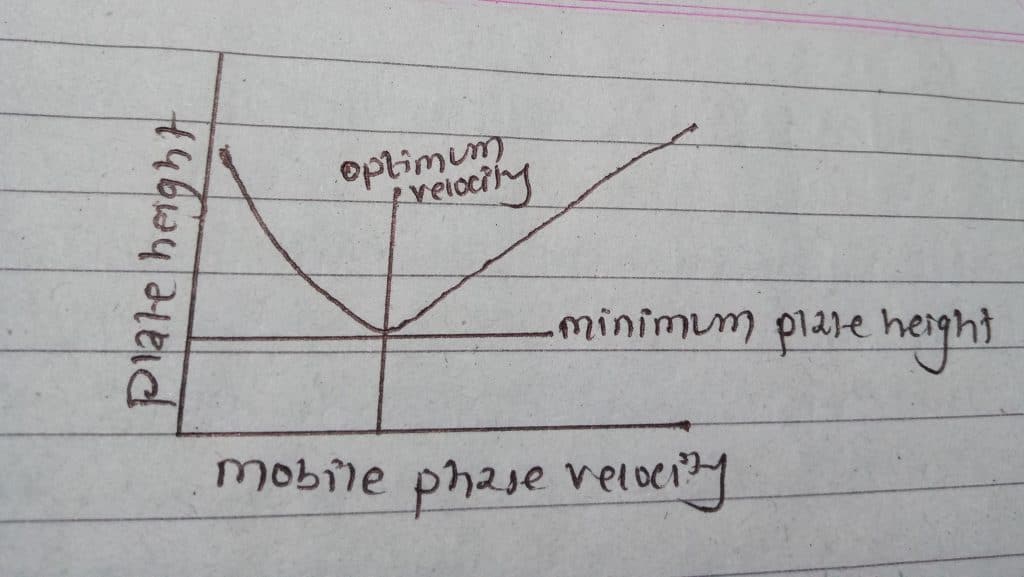
The above plot is Van-Deemter plots useful in determining the optimum mobile phase flow rate.
Thus, from the above studies, band broadening in chromatograph occurs as a result of different kinds of diffusion taking place in the sample movement in the column concerning flow rate. The flow rate must be controlled to get proper/adequate resolution of the peak as the increase in flow rate gives an inappropriate resolution.
From the above effects, Van-Deemter’s equation for HPLC will be
H = A + c u
Similarly, for gas chromatography, it is
H = B/u + c u






