Table of Contents
ToggleAtropisomerism in Organic Chemistry is a type of rotational isomerism or conformational isomerism that occurs in a molecule when rotation about a bond is restricted. Let’s learn about atropisomers(rotamers) in organic chemistry, including their definition and examples, to better understand twisting molecules and chirality.
Atropisomerism Definition
Atropisomerism is defined as the type of isomerism that occurs from the restricted rotation around a single bond, between two sp2–hybridization. Or simply it is the spatial arrangement of the different groups about a single bond called atropisomers and the phenomenon is called atropisomerism. Examples are biphenyls, biaryl ether, benzamides, diaryl amines, etc.
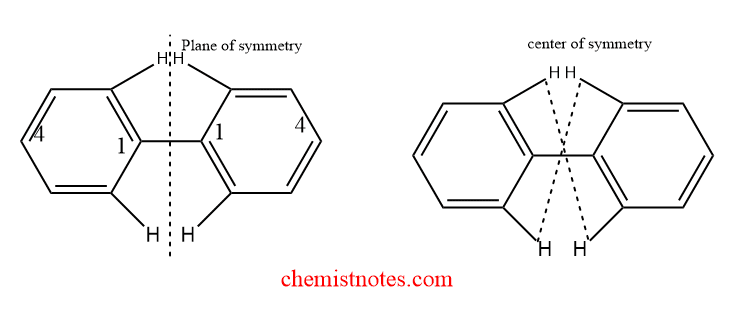
Examples of Atropisomerism
Some examples of atropisomers, are devoid of chiral central but are optically active. These conformers are enantiomers (atropoenantiomers), showing axial chirality when the substituents are achiral; otherwise, they are diastereomers (atropodiastereomers).
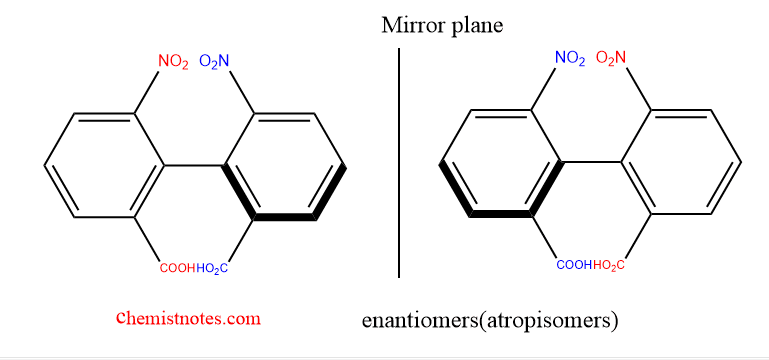
Origin of Atropisomerism
Adams et al. extended atropisomerism, first identified in diphenic acids in 1922 (22JCS614), to heterocycles in 1931. After 80 years, the field is still very active, and it is anticipated to become more significant because atropisomerism is connected to asymmetric synthesis, materials, and biological properties, as well as because new techniques, like low-temperature HPLC, high field, and solid-state NMR, are now available.
Condition of Atropisomerism
In compounds that lack chiral centers, chirality is due to large barriers to double bond rotation, as in allenes, by the molecular framework as a whole, as in spirans, or by a combination of these two factors, as in alkylidenecycloalkanes. In addition to these, chirality can also form in molecules without chiral centers when rotation around a single bond is restricted. Biphenyls are a good example of this.
- On both sides of the axis, different substituents should be present
- Rotationally stable axis
Atropisomerism in Biphenyls
Atropisomerism can be found in many compounds besides properly substituted biphenyls. Biphenyls have two benzene rings that are free to travel through a pivotal bond because the distance between their ortho hydrogens in the planar conformation is approximately greater than twice the hydrogen’s Vander Walls radius. The steric factor does not, therefore, alter the free rotation through the pivotal bond. The molecule lacks a vertical plane of symmetry and can result in atropisomerism when the ortho positions of biphenyls are dissymmetrically substituted.
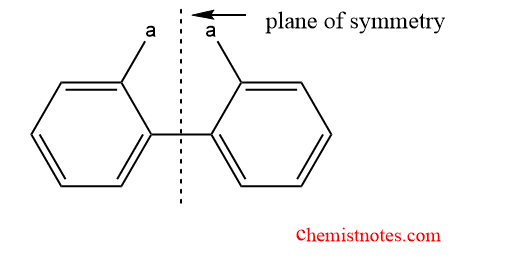
Stereochemistry in Biphenyls
Rotation around a single bond is possible in biphenyls, in contrast to their ortho-substituted counterparts, which are sterically restricted. Biphenyl exhibits conformational isomerism rather than geometric isomerism. Since the individual C2-symmetric isomers of some substituted biphenyls exhibit atropisomerism as a result, they are visually stable. In synthetic chemistry, ligands such as BINAP and similar derivatives are employed. Unsubstituted biphenyl has an equilibrium torsional angle of 44.4°, and the torsional barriers are only 6.0 kJ/mol at 0° and 6.5 kJ/mol at 90°. By using ortho substituents, the barrier is significantly raised; in the case of 2, 2′-dimethyl derivatives, the resistance is 17.4 kcal/mol (72.8 kJ/mol).
Biphenyl is formed when two benzene molecules are linked by a single sp2-sp2 bond. In biphenyl, both benzene rings are situated on the same planes. Therefore, the biphenyl is co-planar in its crystallized condition. It is devoid of chiral centers. A plane and symmetry center is present. It is hence optically inactive. However, the two benzene rings are somewhat twisted in the liquid and vapor phases, which may be due to steric interactions between pairs of hydrogen atoms in the ortho position.
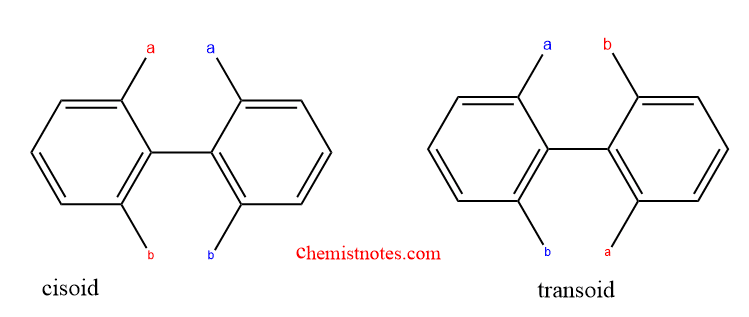
Effect of the substituent in Atropisomerism
There is some sort of symmetry present when the two groups on the phenyl ring’s axis are identical on the same side, and this inhibits atropisomerism because of free rotation around the bond. However, atropisomerism is shown if the two groups are distinct and sufficiently large as a result of the bond’s restriction on rotation.
Numerous physical approaches, including x-ray diffraction, dipole moment measurement, spectroscopic techniques, etc., can be used to explain the existence of restricted rotation in suitably substituted biphenyls.
MCQs/FAQs
What is atropisomerism?
Different spatial configurations of groups are referred to as atropisomerism and are caused by restricted rotation around a single bond.
What is the condition for atropisomerism?
The condition for atropisomerism is a stable axis in rotation and
different substituents are present on both sides of the axis.
Are atropisomers enantiomers?
A non-planar arrangement about an axis is shown by axial chirality in atropisomers. Despite the fact that they lack chiral carbons, they are enantiomers. As a result, the atropisomers can rotate the plane of polarised light..
Do atropisomers have chiral centers?
The steric strain for atropisomers is so high that the molecules are fixed into a single conformation and do not rotate at all. Atropisomerism produces chiral compounds, which are mirror-imaged molecules that cannot be superimposed on one another.
What is atropisomerism in drug discovery?
A chiral axis is produced by restricted bond rotation, which results in atropisomerism, which is stereochemistry. Atropisomers are susceptible to time-dependent chirality inversion by bond rotation, a characteristic that adds complexity and difficulties to the processes of drug discovery and development in drug compounds.






