Table of Contents
ToggleOrganometallic compounds generally denote compounds in which organic groups are linked directly to the metal through at least one carbon atom. Nomenclature, types, synthetic methods, and uses of such compounds have been discussed in this blog.
Define organometallic compounds
Organometallic compounds are defined as those compounds having an M-C bond formed by a metal atom(M) bonding with a carbon atom (C). The organic groups linked to metal through carbon atoms, either directly sigma bond or through Pie-bond. Examples: Organolithium compounds, organozinc compounds, Grignard reagent(RMgX), Wilkinson’s catalyst, and so on.
Compounds such as Ti(OC4H9)4, and Fe(SC5H11)3 are not listed included in the lists of organometallic compounds. In spite of having metal-carbon bonds, some compounds like CaC2, Hg(CNO)2, and Fe(CN)6 -4 are not considered organometallics, which may be due to traditional or some other obvious reason.
- As mentioned earlier, even if the compounds have an M-C bond, these are excluded from the group of organometallic compounds if they show apparently inorganic compound-like properties. For example: Metal carbides and metal cyanides.
- But in contrast, even if the compounds do not have an M-C bond, they are treated as organometallic compounds if they show similar properties to organometallic compounds. For example: Metal hydrides, N2 Coordination complex, etc.
The suffix “Metallic” in the term organometallic represents those elements that are metallic(electropositive) compared to carbon.
Naming organometallic compounds
Based on the IUPAC rules of naming organometallic compounds, the names of a few representative examples of mono and poly-hapto types of organometallic compounds have been discussed below.
For simple compounds, names are written as one word with the name of the organic groups followed by the name of the metal at last.
If there are different groups joined to the same metal then the name is written in alphabetical order. For examples.
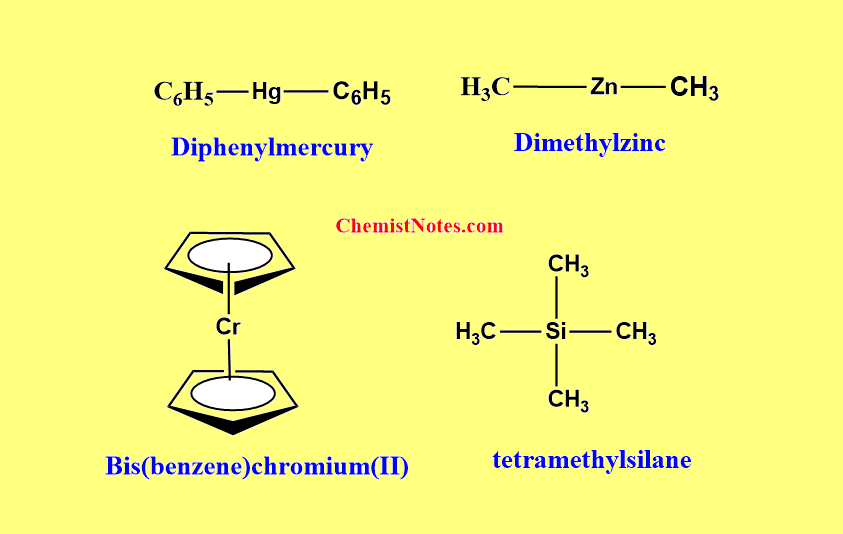
For organometallic derivatives of silicon, an alternative name is given based on their parent hydride.
Types of organometallic compounds
Broadly, organometallic compounds can be classified in two ways.
- Based on Hapticity
- Based on the nature of the metal-carbon bond.
Hapticity represents the number of carbon atoms of the organic moiety/group which are within the bonding distance of the metal.
Classification based on Hapticity
Generally, organometallic compounds are categorized into two types on the basis of hapticity viz. mono-hapto compounds, and poly-hapto compounds.
- Monohapto compounds: In these, the ligand is bonded to a single metal atom through only one of its carbon atoms.
- Polyhapto compounds: In these, the organic ligand has more than one of its carbon atoms within bonding distance of a single metal atom. This includes di-hapto, tri-hapto, and so on.
Classification based on the nature of Metal-Carbon bond
On the basis of the nature of the metal-carbon bond present in organometallic compounds, these are further classified into two types.
- Ionic compounds
- σ-bonded compounds
Ionic compound
- The highly electropositive metals( alkali, alkaline earth metals) and even lanthanides and actinides with electronegativity values nearly 1 or less than 1 form predominately ionic organometallic compounds. There are some exceptions, Lithium, Beryllium, and Magnesium form fairly covalent compounds.
- These are generally colorless, extremely reactive, non-volatile solids, and insoluble in hydrocarbon solvents.
- On heating, these compounds decompose without melting.
- The stability and reactivity of ionic compounds are also affected by the stability of their carbanions. Compounds containing unstable anions are very reactive.
σ-bonded compounds
- In these compounds, a carbon atom of the organic moiety is bonded to the metal by a normal two-electron, two-centered covalent bond.
- These are generally formed by most elements with electronegativity values higher than 1.
- The bond M-C is polarized in such a way that the carbon atom bears a partial negative charge and the metal bears a positive charge.
Electron counts in Organometallic compound
The total number of electrons present in these compounds can be counted by using the 18-electron rule.
Synthesis of organometallic compounds
There are various ways of preparation of metal to carbon bonds that are useful for both main groups and transition metals.
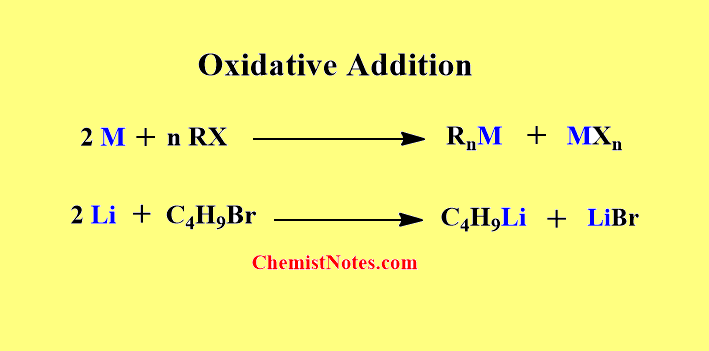
Oxidative addition
Alkyl halide reacts with reactive metals( Li, Na, Mg) to form organometallic bonds.
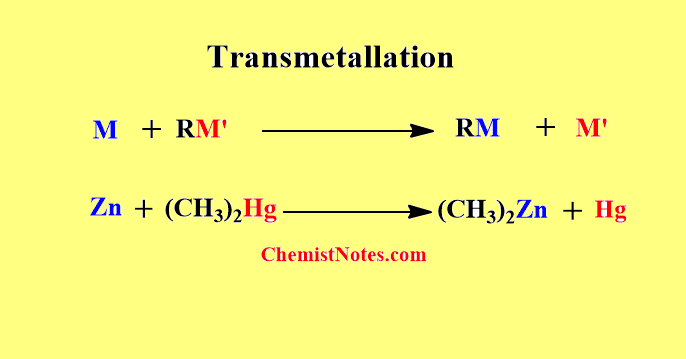
Transmetallation
In this method, one metal reacts with an organometallic compound so that there is the interchange of metallic center and a new organometallic compound is formed.
Metal Exchange
In the metal exchange method, two organometallic compounds are reacted with each other hence, the exchange of metal centers takes place.
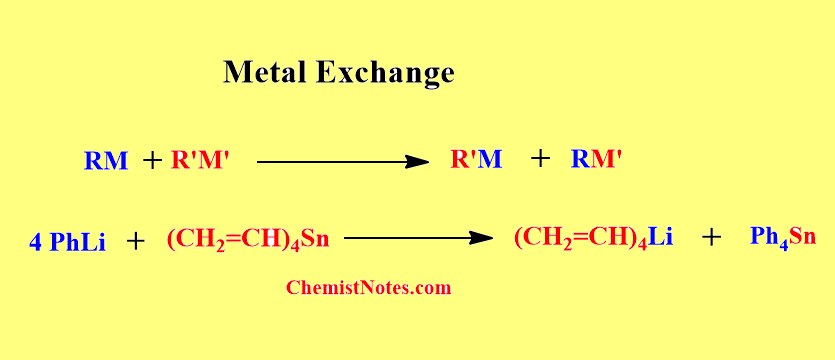
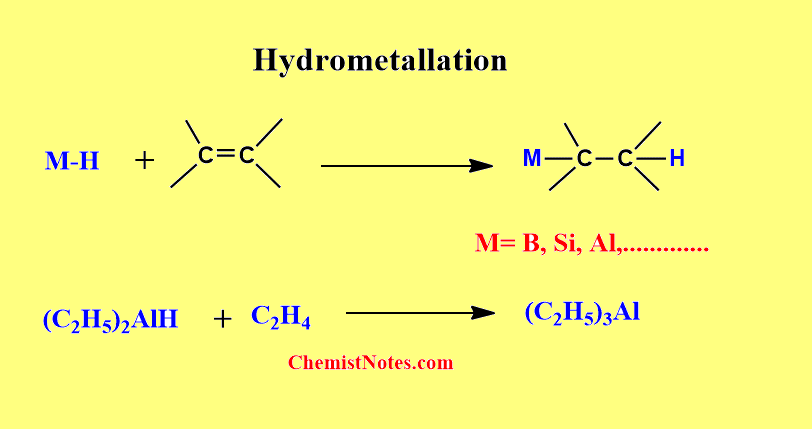
Hydrometallation
When a compound having a metal-to-hydrogen bond(M-H bond) is reacted with a compound having a carbon-carbon double bond or triple bond, a new compound having a metal-to-carbon bond(M-C bond) is formed. This reaction is known as hydrometallation.
Applications of organometallic compounds
Organometallic compounds are very reactive and utilized by taking advantage of their high reactivity.
- Organometallic compounds are largely used as catalysts and organosynthetic reagents in organic synthesis due to their higher reactivity and high reaction selectivity.
- Some are used in chemotherapy.
- Tetraethyllead, which has antiknocking properties, is used in automobiles.
- Dibutyltin dilaurate is used in preventing discoloration or degradation of PVC polymers.
- Grignard reagents are used as a reactive and versatile intermediate for a wide range of organic and organometallic synthesis.
- Some compounds show biological effects. Some biological active compounds are Salvarsan, Merthiolate, and Mercurochrome(antiseptic).
- Some organometallics of mercury and tin are employed as insecticides, pesticides, fungicides, and herbicides. But, compounds of mercury have been banned in many countries due to their toxicity.
- Organotins are used as stabilizers for polyvinyl chloride
FAQs/MCQs:
Is sodium ethoxide an organometallic compound?
Sodium ethoxide is not an organometallic compound because this compound has no direct carbon to Sodium bond.
How are organometallic compounds classified?
These are generally classified on the basis of the nature of metal-to-carbon bonds.
How to count electrons in organometallic compounds?
Electrons in these compounds can be counted by using the 18-electron rule.






