Table of Contents
ToggleRetrosynthetic analysis is an imaginative procedure in which the target molecule(TM) is broken down or disassembled into less complicated, simpler, and more readily available starting material.
Retrosynthetic analysis is based on the systematic breakdown of the target molecules to produce a more basic structure that can be produced by known or plausible processes.
Define retrosynthetic analysis
The retrosynthetic analysis is an approach in synthetic chemistry in which a target molecule is broken into simple easily available materials. This type of process in which the target molecule is broken down logically into simple easily available or commercially available molecules is known as the disconnection approach.
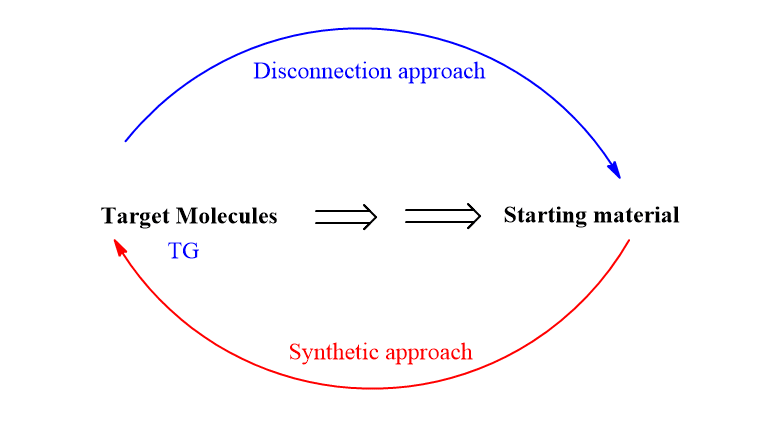
Strategic bonds are those bonds in a molecule that, when disconnected in a retrosynthetic study, result in a considerable structural simplification. The products of disconnection are synthons, which may be anionic (or carbanion), cationic fragments( or carbocation), or free radicals. Behind synthons, real molecules should exist, denoted as reagents or synthetic equivalents.
The general scheme of retrosynthetic analysis is given below:
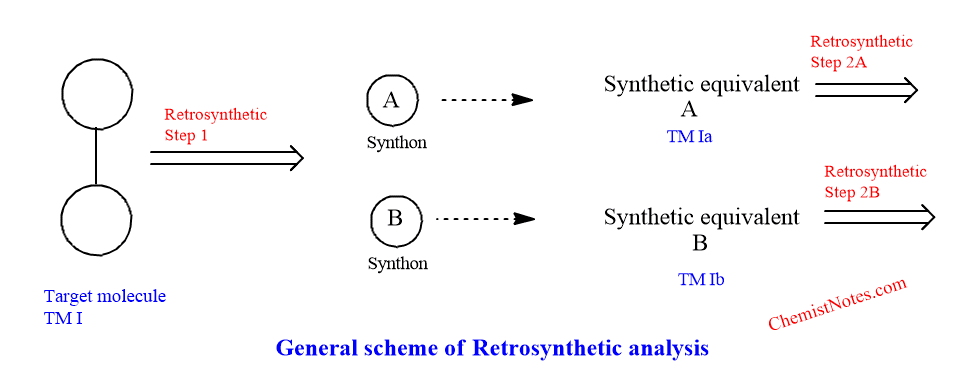
In the diagram above, the waved line and bent arrow over the lines stand-in for the important c-c bonds and the point of disconnectivity or strategic bonds, respectively. The broad arrow illustrates how the target molecules (TM I) are disconnected from the charged species ( synthons A and B). The conceptual relationship between the synthons and actual chemicals, reagents, or synthetic equivalents is then shown by the double dashed arrows.
The synthetic equivalent is denoted as TM Ia and TM lb and is also known as the target molecule of the second generation. Their disconnection represents the second retrosynthetic steps 2A and 2B and this process continue until simple, commercially available compounds are reached. Thus any new target molecules should have more easily available synthetic equivalents.
It should be noted that the number, type, and relative position of the functional group in the target molecules are what essentially control the disconnection process.
The basic property of the c-c bond, electronic structure, and electronic charges of the fragments that are produced when this bond is broken is associated with the most significant retrosynthetic rule. According to the rule, the disconnect should use the correct mechanism.
When a c-c bond is completely disconnected to obtain synthons with a charge stabilized, such disconnection is termed logical disconnection or the disconnection that follows the correct mechanism. Thus, synthons resulting from correct disconnection accommodate a stabilized charge negative or positive or an electronic sextet in carbene.
The basic principle for good disconnection:
- Disconnection should follow the correct mechanism
- Disconnection should follow the maximal simplification of the target molecule.
- By the sequences of disconnection, we shall arrive at simple, easily available starting materials.
There are main three transformations of the functional group viz; interconversion (FGI), Elimination(FGE), and Addition (FGA).
Retrosynthetic analysis examples
What is the basic difference between synthesis and retrosynthesis? In synthesis, target molecules are synthesized by using simple commercially available materials. For example, Cyclohexene can be synthesized by using diene and ethene.
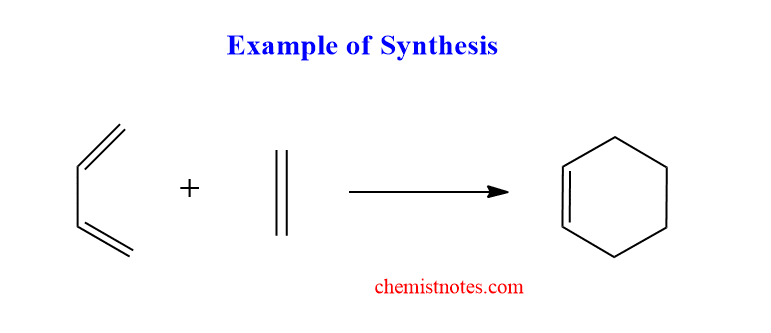
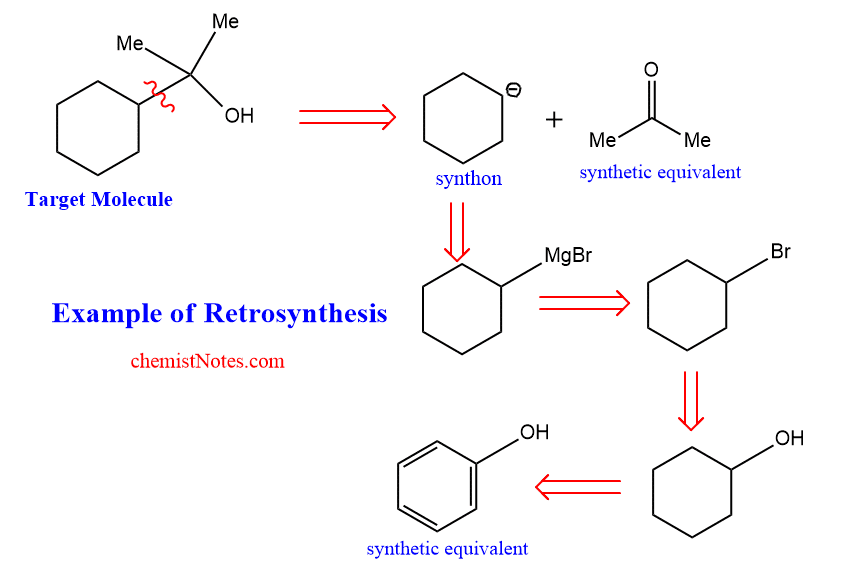
But Retrosynthetic is exactly the opposite of the synthetic process, in which complex target molecules are broken into simple, commercially available materials.
Retrosynthesis analysis youtube
FAQs:
What is retrosynthetic analysis?
Retrosynthetic analysis is an imaginative procedure in which the target molecule(TM) is broken down or disassembled into less complicated, simpler, and more readily available starting material.
References:
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- M. B. Smith, Organic Synthesis, (3rd Edition), McGraw-Hill Companies, 1994.
- S. Warren, Organic Synthesis, The Disconnection Approach, Wiley, New York, 1982.






