Table of Contents
ToggleThe Grignard reaction is an organometallic chemical reaction that involves the addition of alkyl, allyl, vinyl, or aryl-magnesium halides (Grignard reagent) to a carbonyl group in an aldehyde or ketone. This process is crucial in the creation of carbon-carbon bonds.
Grignard’s reaction with aldehyde and ketone
The reaction of the Grignard reagent with aldehyde and ketones is one of its most important applications. The Grignard reagent’s carbon-magnesium bond is a strongly polar bond, with carbon being negatively related to electropositive magnesium. With the addition of carbonyl compounds, the organic group is attached to carbon and the magnesium group is bonded to oxygen. The result is the magnesium salt of the weakly acidic alcohol, which is easily transformed into alcohol by adding the stronger acid, water.
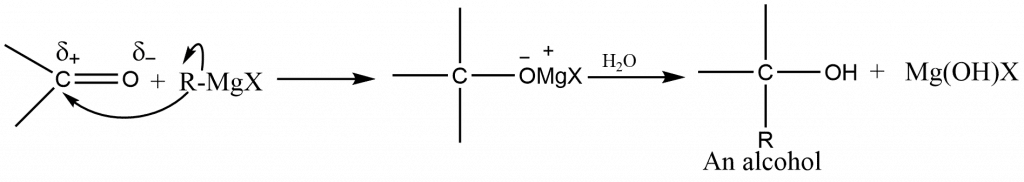
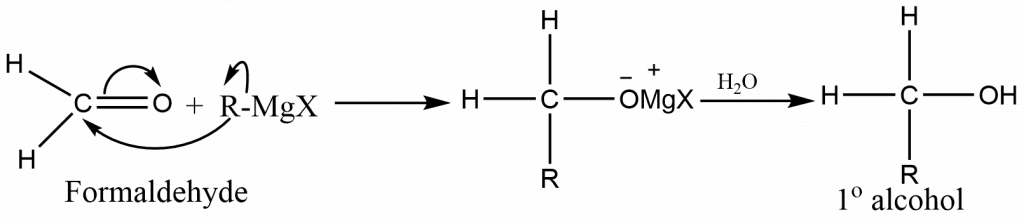
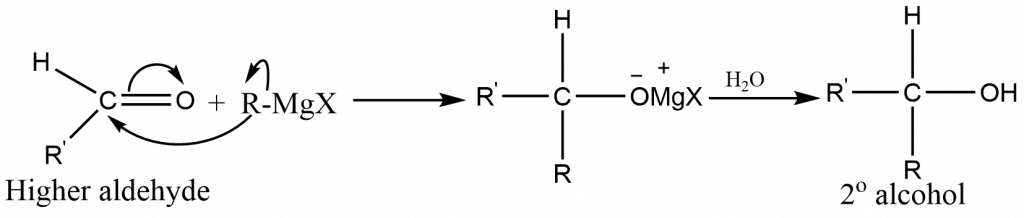
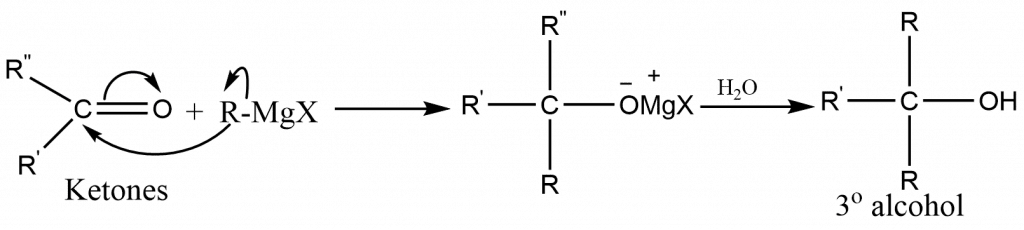
The type of alcohol formed from a Grignard synthesis is determined by the type of carbonyl compound used:
Formaldehyde (HCHO) yields primary alcohol
Other aldehydes (RCHO) yield secondary alcohol
Ketones (R2CO) yield tertiary alcohol
Grignard synthesis of carboxylic acid
The Grignard synthesis of a carboxylic acid is performed by either bubbling gaseous CO2 into the ether solution of the Grignard reagent or pouring the Grignard reagent over crushed Dry ice (solid CO2); in the latter technique, Dry Ice acts as both reagent and cooling agent. The Grignard reagent adds to the carbon-oxygen double bond in the same way that aldehyde and ketones do. The product is the carboxylic acid magnesium salt, from which the free acid is liberated after treatment with mineral acid.
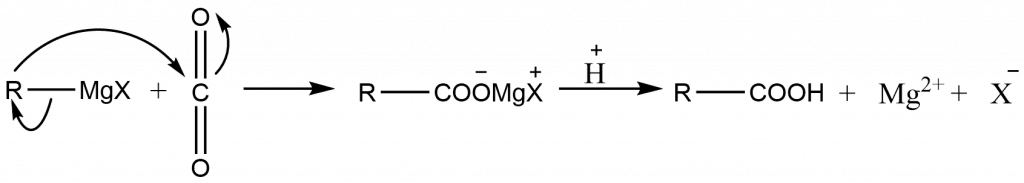
FAQs
What is the Grignard reaction?
The Grignard reaction is an organometallic chemical reaction that involves the addition of alkyl, allyl, vinyl, or aryl-magnesium halides (Grignard reagent) to a carbonyl group in an aldehyde or ketone.
Refrences
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987






