Table of Contents
ToggleWe generally get amused by the interesting fact behind some unique things, no wonder it’s all because of Chemistry. Some of the interesting facts related to Chemistry are:
Fun chemistry question:
What happens when one molecule of barium is treated with two molecules of sodium?
Ans: It’s simple as chemistry: it BaNaNa haha ( in reality there is no such reaction happen)
What happens when one molecule of cobalt is treated with two molecules of iron?
Ans: Simple it CoFFee (got it haha)
One question for the audience
What happens when carbon, helium, manganese, iodine, sulfur, and tennessine were treated with nitrogen, oxygen, tellurium, and sulfur?
Hint ( you are reading right now)
Now, let’s start with amazing, mind-blowing facts related to chemistry:
1. Why does wine glass have a thinner and more slender stem?
In order to keep wine chilled, wine glasses have tall, slender stems. Long stems on wine glasses make it possible to hold the glass in your fingers without touching the wine, which warms the wine. As was previously mentioned, the wine gets hotter when a glass is held by its bowl.

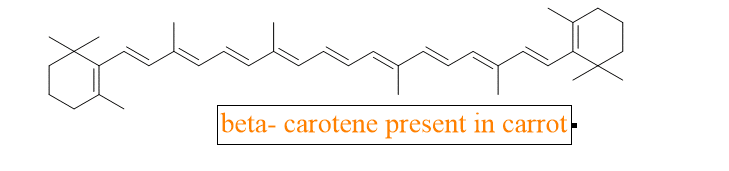
2. Why is the color of the radish white while the carrot is orange in color?
All of this is attributable to the procedure known as UV spectroscopy. The reason there isn’t any such substance present in white radish is that the pigment or chromophore known as beta carotene present in carrots has a number of double bonds that are in conjugation and the presence of multiple pi bonds and their interactions within the molecule can affect the energy gap between the molecular orbitals and require low energy to cross the barrier. As a result, they have longer wavelengths and fall in the visible range and we see them as orange-colored.
3. Why do cotton clothes easily get wrinkled after first washing?
Cotton clothing is known to be composed of cellulose and polymer-bonded. Fabrics with hydrogen bonds are particularly absorbent; as a result, when moisture is added, water permeates and breaks these bonds. The wrinkles that developed when the shirt was wet are fixed in place as new hydrogen bonds form as the moisture evaporates.
4. Why is there a gap between the hairbrush, and comb?
It’s natural and important for there to be a slight space between the toothbrush handle and the brush head. For the correct quantity of vibrations to be produced, the brush head must be mobile.

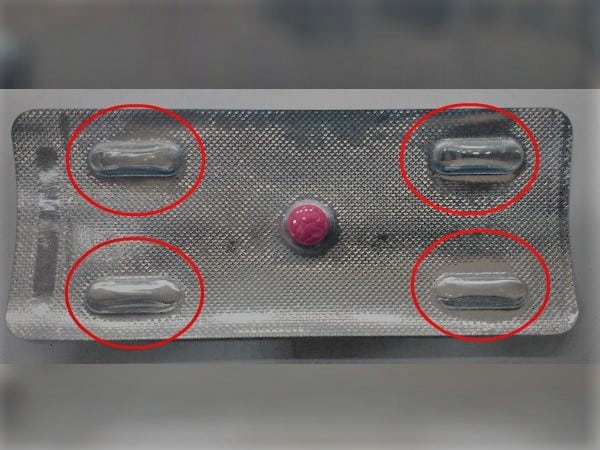
5. Why is there a gap in medicine tablets?
We always ask ourselves, “Why are they tricking us with an empty space?” whenever we see a strip of medicine. When you first saw it, you could have assumed that the corporation had neglected to restock the medication, but that is not the real cause of the vacant spot. There are a few reasons for that:
- Most people think that by pumping vacuum or inert gases inside the empty areas, the empty gaps are made to strengthen the tablet’s inertness.
- The business includes these extra spaces in order to maintain the regular size of the packing boxes and avoid incurring additional costs.
- The content must be printed on the reverse of medication strips that only contain one pill. This adds even another explanation for why there are these empty places.
- These additional empty areas aid in preventing drug breakage and spillage when being transported. This is among the most significant justifications for why these empty gaps exist.
6. Why suddenly the solution became pink-white titrating acid and base with the indicator phenolphthalein?
The organic acid phenolphthalein is quite weak. Its ion (Ph-) is pink in color, whereas its undissociated molecule (HPh) is colorless. Phenolphthalein has a pH range of 8–9.8. Phenolphthalein is used as an indicator in acid-base titration. The solution turns pink from colorless as the concentration of OH ions slightly rises, indicating the endpoint.
7. Why is there no j element in the periodic table?
J is not used in many other languages, including Latin. This is another justification for J’s absence from the periodic table. Despite being known as jod, the international element symbol for iodine is I. To honor the Japanese RIKEN team, the 113th element was going to be given a name that begins with the letter J.
8. Water expands, when freezes, unlike other substances.
Generally, things shrink when they are cold. Due to the fact that temperature characterizes atomic vibration, the greater vibration causes the atom to occupy more space, leading to expansion. The one exception is water. The ice takes up more space when it is frozen, albeit vibrating less. The peculiar structure of the water molecule is responsible for this.
With two hydrogen atoms at an angle for Mickey’s ears and one oxygen atom in the center for the face, the water molecule resembles the cartoon character. The water molecule has an open structure with a lot of room because of how oxygen and hydrogen connect to one another. Energy is released when water freezes because many extra-strong connections can be formed. However, it does occupy more room. Ice expands as a result of freezing. The fact that hot water freezes more quickly than cold water is another fascinating fact worth highlighting.
9. Why do onions make us cry?
When the onion is chopped, certain substances called lachrymators are released, irritating the lacrimal glands and the nerves that surround the eyes. Methionine and cystine, two members of the amino acid family, make up these molecules.
10. Why do, the air hostesses give us chocolates during the flight?
To maintain a balanced atmosphere, that is. As you can see, the inner ear portion of the ear decreases as height increases. Therefore, some air must escape from the mouth in order to maintain the eustachian tube’s balance, and more air will exit the mouth as we continue to eat the chocolate.