Table of Contents
ToggleThe shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in the inner shells. When there are more inner electrons, they shield the outermost electron from the nucleus and neglect the nuclear attraction to some extent. This is called the “screening” or “shielding” effect. According to the shielding effect, electrons nearer the nucleus “shield” electrons farther from the nucleus’ positive charge.
Because electrons in a particle have different extents of attraction power, the shielding effect also represents the reduction of the particle’s tremendous atomic charge on the electron cloud. Shielding effects are illustrated by the nuclear fission reaction. Electrons are pulled off the nucleus, just like in nuclear fission.
What is shielding effect?
In multi-electron atoms, the electrons present on the valence shell are attracted toward the nucleus. At the same time, these valence electrons are also repelled by the electrons present in the inner shells (these inner electrons are also called intervening electrons). Due to this repulsive force, the actual nuclear attraction on the valence shell decreases. A decrease in nuclear attraction on the valence shell due to the presence of electrons in the inner shells is called the shielding effect or screening effect.
The magnitude of the screening effect depends on the number of electrons present in the inner shells. The greater the number of inner electrons, the higher the screening effect. The magnitude of the shielding effect determines the extent to which the nucleus attracts the valence electrons. The greater the shielding effect, the lower the nuclear attraction on the valence shell.
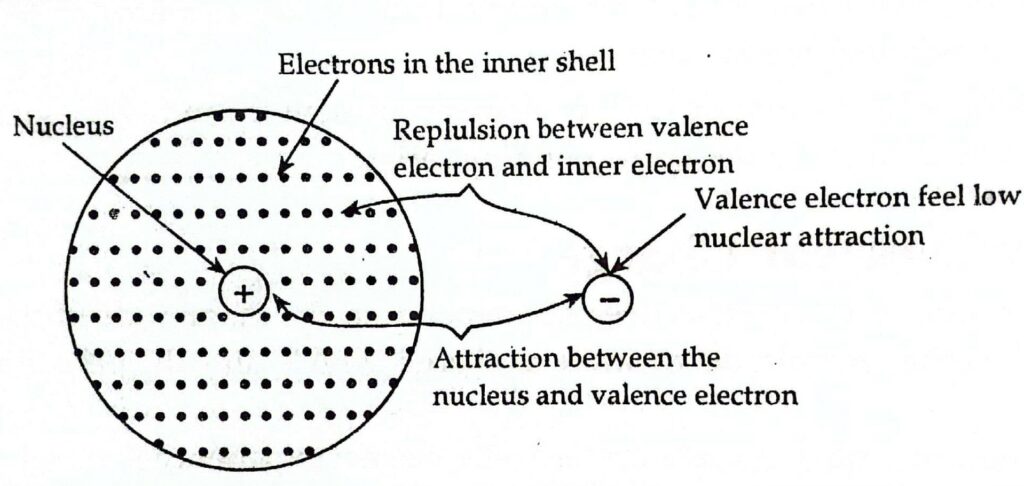
Factors affecting shielding effect
The following factors determine the magnitude of shielding effects:
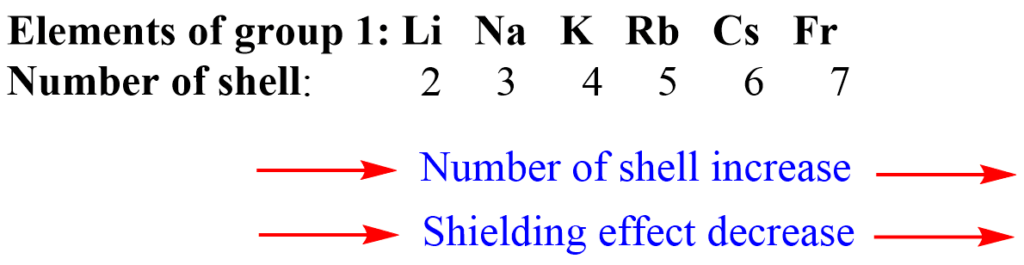
1. Number of shells: The greater the number of shells present between the nucleus and the valence shell, the higher will be the shielding effect.
2. Nature of orbital: According to wave mechanics, the penetration power of electrons in different orbitals of a given shell decreases in the order:
Penetration power: ns > np > nd > nf
Above order represent that the s-orbital lie nearest to the nucleus. Hence electrons in the s-orbital have maximum ability to shield the nucleus. In other words, s-electrons can block the nuclear attraction to the highest extent. As the penetration power of other orbitals decreases, they lie far away from the nucleus. Hence their shielding effect decreases in the same order i.e.,

Application of Shielding Effect
- The concept of the shielding effect explains the variation of atomic radius, ionization energy, etc. along the period from left to right along a group from top to bottom in the periodic table.
- Shielding effect explain the cause of the vast difference in ionization energy between noble gases and alkali metals.
- It explains the irregular variation of atomic radius and ionization energy of elements present in group IIIA (13) [i.e. B, Al, Ga, In, and Tl]
- It also explains the cause of similar atomic radii of the elements of the second and third transition series belonging to the same group.
- It also explains why the atomic radii of elements present in the lanthanide series and actinide series are almost the same in their corresponding series.
Effective Nuclear Charge
The entire property of atoms does not depend on the magnitude of nuclear charge in the nucleus but on the magnitude of nuclear charge which directly influences the valence electrons.
In poly-electronic atoms, the valence electron does not experience the total nuclear charge. Some of the nuclear charges are shielded by the intervening electrons. Consequently, the nuclear attraction on the valence shell decreases. The magnitude of nuclear charge which is felt by the valence electron is called effective nuclear charge. It is denoted by Zeff and given by the relation,
Zeff = Z − σ
Where, Z = number of nuclear charges i.e., atomic number
σ = Shielding constant which is a measure of the shielding effect caused by intervening electrons
The shielding constant and effective nuclear charge can be calculated quantitatively using Slater’s rule.
Shielding Effect Video
References
- Rayner-Canham, Geoffrey (22 December 2013). Descriptive inorganic chemistry. Overton, Tina (Sixth ed.). New York
- Shriver, Duward (2006). Inorganic Chemistry (4th ed.).






