Table of Contents
TogglePseudohalide ion
There are some univalent negatively charged groups that are composed of two or more electronegative atoms and resemble halide ions in some aspects. Such univalent negatively charged ions are called pseudohalides. Examples of pseudohalides ions are given below:
- Cyanide ion(CN–)
- Isocyanide ion(NC–)
- Fulminate ion(ONC–)
- Cyanate or oxy-cyanide ion(OCN–)
- Isocyanate ion(NCO–)
- Thiocyanate ion(SCN–)
- Selenocyanate ion(SeCN–)
- Azide ion(N3–)
The covalent dimers of the pseudohalide ions are called psedohalogens or halogenoids. Some examples of pseudohalogens are Cyanogen(CN)2, Oxy-cyanogen(OCN)2, Thiocyanogen(SeCN)2 etc.
Similarities between halide and pseudohalide ions
Some of the important similarities between halide ions and pseudohalide ions are given below.
- Formation of ionic as well as covalent compounds: Like halide ions, pseudohalide ions also form ionic as well as covalent compounds. For example, AgCl, and AgCN are ionic compounds while ICl and ICN are covalent compounds.
- Oxidation of hydracids: HX acids(X= Cl–, Br–, I–) can be oxidized to free halogen(X2). Similarly, HY acids( Y=SCN, CN, etc) can also be oxidized to free pseudohalogens (Y2).
- Formation of complex ions: Like halide ions, pseudohalide ions also form complex ions with transition metal ions. Examples:[FeF6]3- and [Fe(CN)6]3-.
- Formation of interpseudohalogen and interhalogen compounds: Halide ions can combine together to form interhalogen compounds like ClF, ICl, IBr, etc. Similarly, Pseudohalide ions can also combine together to form interpseudohalogen compounds like CN.N3, and CN.SCN etc.
- Formation of insoluble salts: Like halide ions, pseudohalide ions give insoluble salts with Ag+, and Hg+ cations.
- Like halide ions, pseudohalide ions also have more than one pair of electrons and hence can coordinate with two metal ions simultaneously.
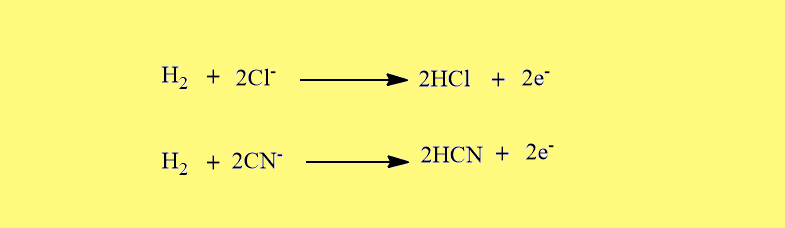
- Like halide ions, Pseudohalide ions also combine with H2 to form monobasic hydracids.
Difference between halide and pseudohalide ions
- The hydracids formed by the pseudohalides ions in combination with hydrogen are relatively weaker than the analogous hydracids formed by the halide ions. For example; HCN is a very weak acid while HCl is a very strong acid in an aqueous medium.
- Pseudohalide ions are stronger coordinating ligands than halide ions and hence the complexes formed by pseudohalide ions are low spin while those formed by halide ions are high spin. For example; [Fe(CN)6]3- is a low spin complex while [FeF6]3- is a high spin complex.
- Pseudohalide ions, being made up of two heteroatoms can function as ambidentate ligands. But halide ions have no tendency to act as ambident ligands.
Similarity between halogens and pseudohalogens
There are some points showing similarities between the two classes of compounds.
Volatile and dimeric nature
Like halogens, pseudohalogens are also dimeric and volatile in the free state. But with, the exception of polymeric thiocyanogen.
Isomorphous nature
Pseudohalogens are isomorphous to halogens when in the free or solid state. For example; Cl2 is isomorphous to (CN)2 and similarly, Br2 is isomorphous with (SCN)2.
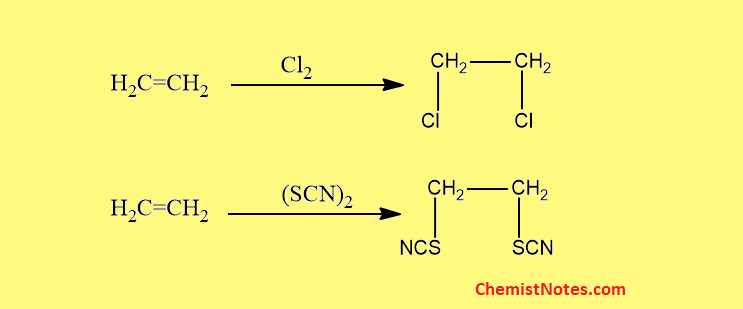
Addition to ethylenic double bonds
Like halogens, pseudohalogens also add to ethylenic double bond linkage.
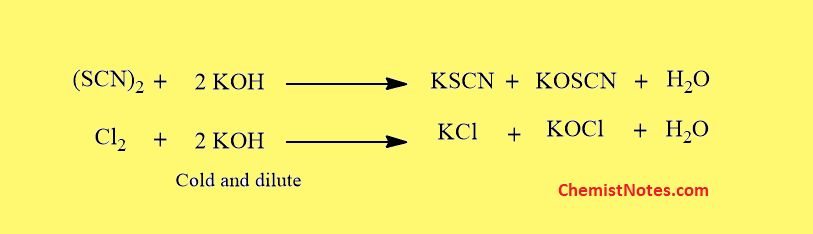
Reaction with Alkali
Like halogens, pseudohalogens also react with alkalies.
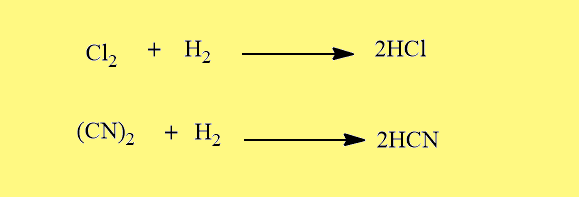
Formation of monobasic hydracids
Like halogens, pseudohalogens also combine with H2 to form monobasic hydracids.
Dissimilarities between halogen and pseudohalogens
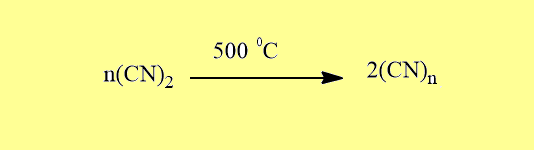
Pseudohalogens undergo polymerization and form polymerization series but halogens have no tendency to undergo polymerization.







One Response
Help to write a note